Human Subjects Division
Changing External IRB Studies
Overview
HSD only requires limited ongoing information about externally reviewed studies. Review the CHECKLIST: External IRB, UW Researchers for more information about when studies that are being reviewed by a non-UW IRB require a study update. Contact hsdrely@uw.edu with any questions.
Review the table below for the most common changes to external IRB studies.
| Change | How to Initiate |
|---|---|
| Update UW PI | Review Change Study PI Instructions |
| Update Study Team (required to change access to the Zipline application) | Review Update Study Team Instructions |
| Change Reviewing IRB | Contact hsdrely@uw.edu to initiate this process |
| Planning to Implement E-Consent | Review Update Study Details instructions
NOTE: You also need to Create Site Modification if you need to upload a new or revised SUPPLEMENT: Other REDCap Installation and/or TEMPLATE: Other E-Signatures Attestation Letter on the Local Site Documents page for a multi-site external study. |
| Report Study Closure | Contact hsdrely@uw.edu to initiate this process |
How to Update Study Details
Multi-site external IRB studies (includes most studies created after 1/7/2020) must submit a site modification to make changes to the local study team, update Local Funding Sources, or update the Local Site Documents.
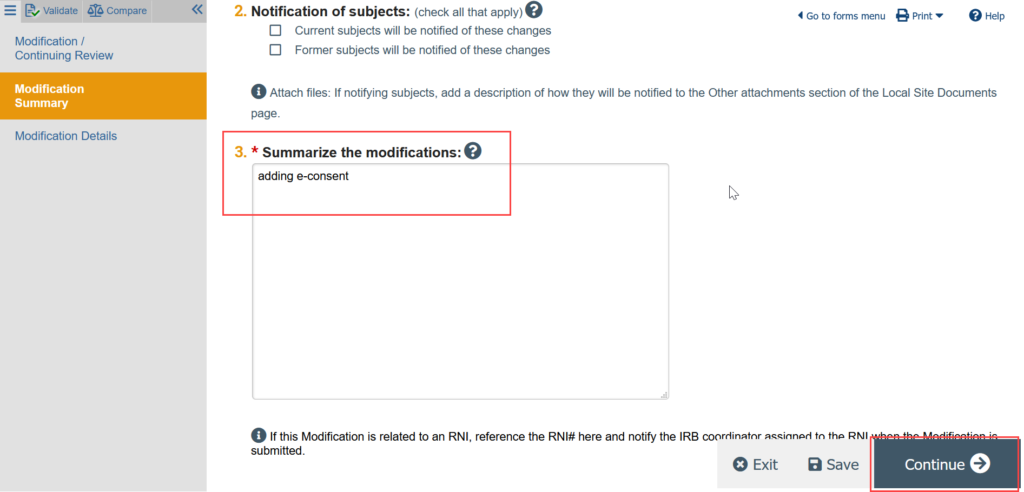
- In the study workspace, click Update Study Details
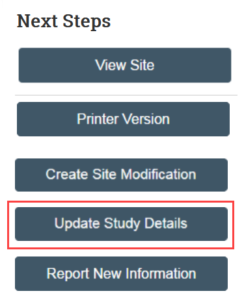
- Provide a summary of the update (for example, adding e-consent)
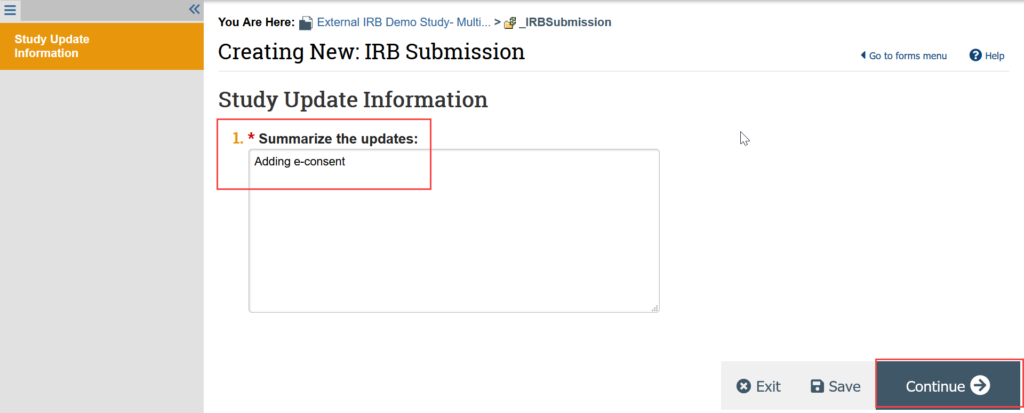
You are now in a draft version of the study SmartForm and should be on the Basic Study Information page. Update the draft version of the study to reflect any changes being made by making all needed revisions to the SmartForms.
ADDING E-CONSENT:
- Update the revised REQUEST: External IRB Review form on the External IRB SmartForm page. If you completed a REQUEST: External IRB Review that did not include questions about E-Consent, you will need to download and complete a new REQUEST: External IRB Review form.
- If needed, upload the SUPPLEMENT: Other REDCap Installation or TEMPLATE: Other E-Signatures Attestation Letter on the Local Site Documents page. If the Local Site Documents page is not available, your study is a multi-site external study and you must submit a separate site modification to add the SUPPLEMENT: Other REDCap Installation and/or TEMPLATE: Other E-Signatures Attestation Letter.
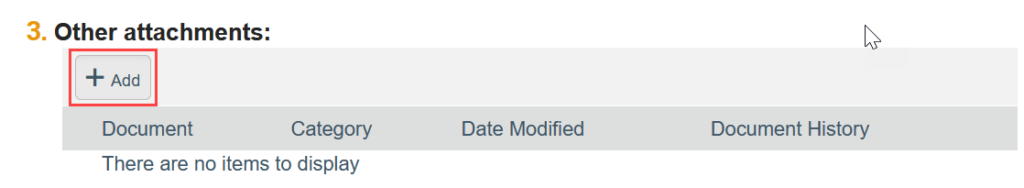
To Add a Document:
- Click Add
- Select the new document from your computer
- Click OK in the Edit Attachment window
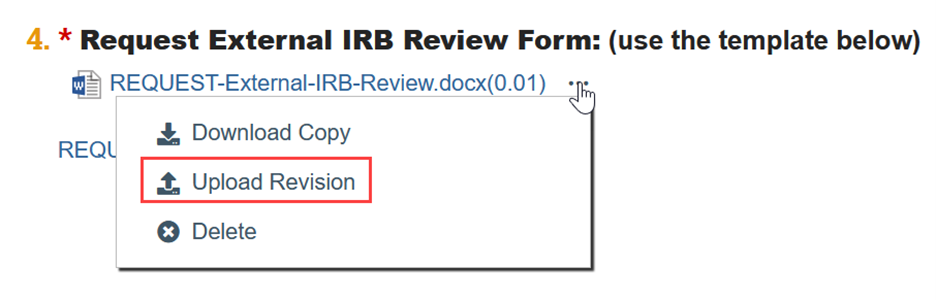
To Update a Document:
- If needed, click the ellipsis by the document
- Click Upload Revision or Update
- Select the revised file from your computer
- Click OK in the Edit Attachment window
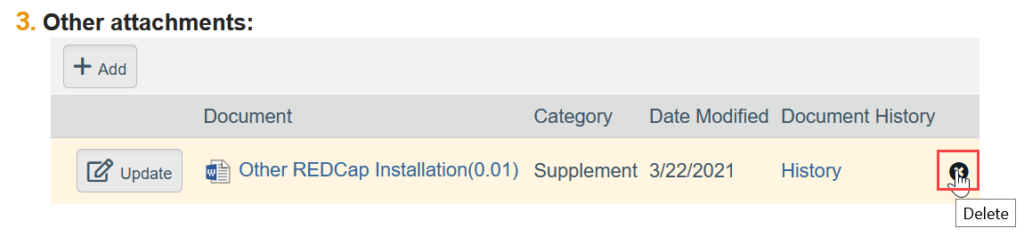
To Remove a Document:
- Click the X by the document
Click Save and Exit out of the draft SmartForm once your changes are complete
• HSD staff will finalize the update after your change has been assessed.
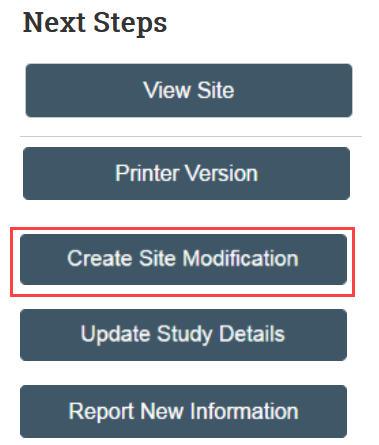
How to Create Site Modification
This activity is only available for multi-site external studies (includes most external studies created after 1/7/2020).
- In the study workspace, click Create Site Modification
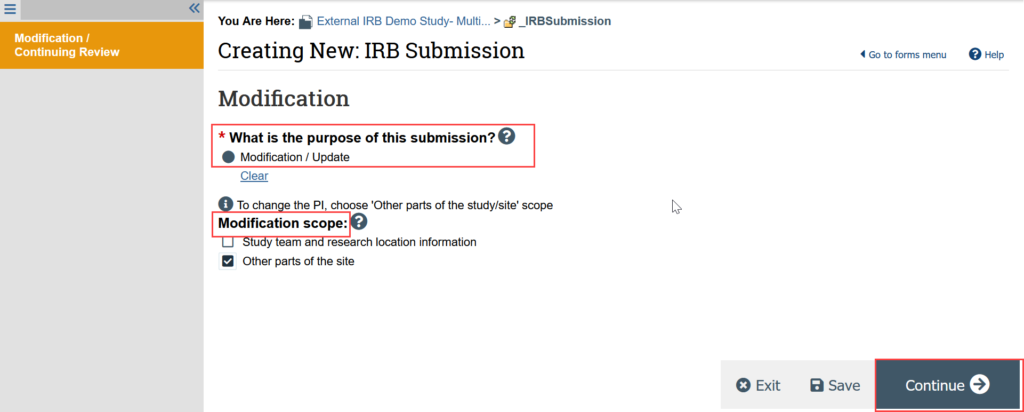
- Indicate that the purpose of the submission is Modification/Update
- Indicate the modification scope (can select both options)
- STUDY TEAM CHANGES: See Update Study Team for detailed instructions
- ADDING E-CONSENT: Select “Other Parts of the Site”
- Add a summary of the changes
You are now in a draft version of the site SmartForm and should be on the Basic Local Site Information page. Update the draft version of the site to reflect any changes being made by making all needed revisions to the SmartForms.
ADDING E-CONSENT:
A site modification is only needed if you need to upload the SUPPLEMENT: Other REDCap Installation and/or TEMPLATE: Other E-Signatures Attestation Letter on the Local Site Documents page.
If you haven’t already done so, Update Study Details to update the revised REQUEST: External IRB Review form on the External IRB SmartForm page.
To Add a Document:
- Click Add
- Select the new document from your computer
- Click OK in the Edit Attachment window

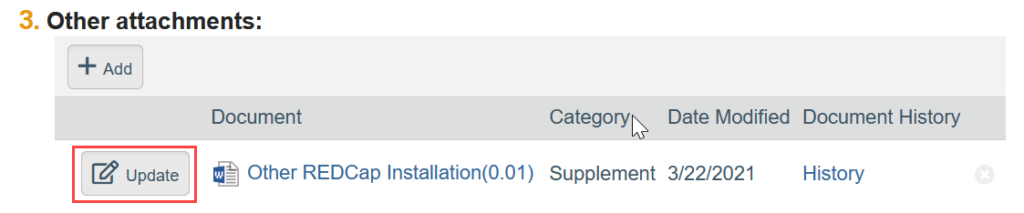
To Update a Document:
- If needed, click the ellipsis by the document
- Click Upload Revision or Update
- Select the revised file from your computer
- Click OK in the Edit Attachment window
To Remove a Document:
- Click the X by the document

- Click Save and Exit/Finish to exit the SmartForm
- Complete the Submit activity to send the modification to HSD for review