UW Research
Research Involving Benaroya/Virginia Mason
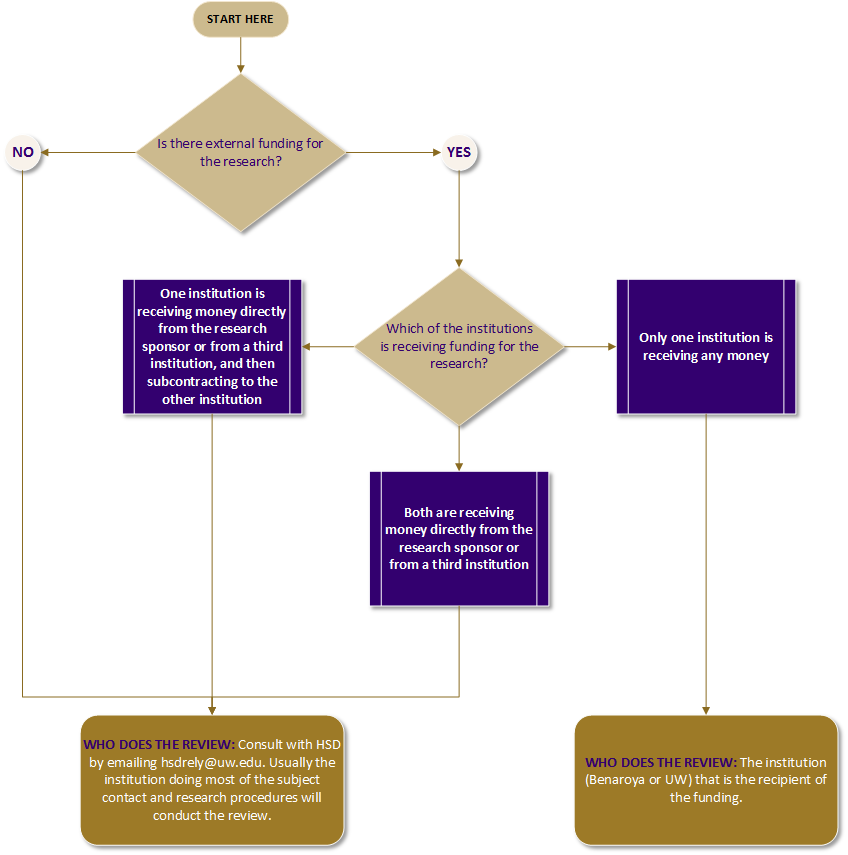
Research involving two institutions normally requires review by each institution’s IRB. To avoid this “dual review” and the extra work it requires from researchers, Benaroya Research Institute (BRI) and the UW IRB have a reliance agreement that describes the circumstances in which one of the IRBs will conduct the review on behalf of both institutions.
Use the flow chart below to determine which IRB should do the review. If you have any questions, consult HSD at hsdrely@uw.edu or BRI at CRP@Benaroyaresearch.org.
Before you begin
- Assess whether both institutions are engaged in the research. If only one institution is engaged, you should submit to that institution’s IRB. Consult with HSD at hsdrely@uw.edu to identify whether UW is engaged.
- Assess whether another IRB is the right option. For example, most industry-sponsored research at UW should be reviewed by an independent IRB (for example, Advarra) and some cancer-related research should be reviewed by the Fred Hutch IRB or the NCI IRB. Review the information about identifying the correct IRB if you are unsure.
What to do if Benaroya will do the review
- Submit an External IRB Application in UW’s Zipline system to request HSD’s formal authorization for BRI to review the research. HSD will evaluate your request and provide you with a written authorization letter.
- The BRI investigator should prepare and submit an application to the BRI IRB, using BRI’s forms and process. Be sure to attach the HSD authorization letter; the BRI IRB will not review the application on behalf of UW without this letter.
- After the BRI IRB has approved UW’s participation, send the approval letter to hsdrely@uw.edu.
- Obtain all other applicable ancillary reviews (consult the Checklist of Researcher Responsibilities for Externally Reviewed Studies) from the usual IRB offices and BRI, as needed. Examples: Financial Conflict of Interest (FCOI) review; Radiation Safety review.
- Exception: BRI will provide Genomic Data Sharing (GDS) certification (if necessary) on behalf of both institutions.
What to do if UW will do the review
- Submit a UW IRB application in the Zipline system. Be sure to:
- Describe all involvement of BRI in your application (the Zipline SmartForms as well as the IRB Protocol form that is uploaded).
- Follow the instructions for the UW Reviewing for Other Institutions pathway.
- On the Study-Related Documents SmartForm: Upload a completed SUPPLEMENT: Multi-site or Collaborative Research to Question 3.
- Submit a request to rely on the UW IRB to the BRI IRB using BRI’s forms and processes. Contact CRP@Benaroyaresearch.org for assistance or instructions. BRI will evaluate your request and provide you with written authorization for the UW IRB to do the review. Include this written authorization in your application to UW or email to hsdrely@uw.edu.
- The UW IRB will review and approve the overall protocol. This is not yet approval for the involvement of BRI. After the UW IRB has approved the overall protocol, HSD’s Reliance Team will work with you to approve the addition of BRI to the UW application.
- After UW IRB approval for BRI has been obtained, you are responsible for:
-
- Informing the BRI IRB of UW’s approval for BRI.
- Informing BRI investigators about any conditions of the IRB approval.
- Informing BRI investigators about their obligation to comply with UW IRB reporting requirements about noncompliance, complaints, problems, etc., although such reports must be submitted by you to the UW IRB.
- BRI investigators remain responsible for obtaining any applicable ancillary reviews from BRI. Examples: Financial Conflict of Interest (FCOI) review, Radiation Safety review.
- Exception: UW will provide Genomic Data Sharing (GDS) certification (if necessary) on behalf of both institutions.
BRI or UW IRB Decision Tree