December 2, 2025
For the Record- December 2, 2025: Dissolution of Committee B, Reminders
In this Issue:
- Dissolution of Committee B & Department Re-Assignments
- Zipline Downtime
- Revised Data Security Guidance
- Upcoming Webinars on OnCore Reporting for Diversity in Clinical Trials
- Longer Turnaround Times for External Authorizations for Clinical Trials
Dissolution of Committee B & Department Re-Assignments
Reminder
In the November 4 HSD Newsletter, we announced that HSD will be disbanding IRB Committee B effective December 9th, 2025.
HSD’s review system is based on pairing each academic department with a specific IRB committee and supporting team. This dissolution will require some shifting of new and existing IRB protocols.
New Submissions
All departments currently assigned to B will be reassigned to one of the remaining three review teams (i.e., A, D, and J). New submissions received on or after December 9th will be assigned to their new team in the Zipline system.
Existing Studies
Existing studies will be automatically re-assigned to their new IRB committee and associated team in Zipline by HSD.
Who is my new team and how do I contact them?
All information about new team assignments and contact details will be available on our website on December 9th.
If you have any specific questions, please contact hsdinfo@uw.edu.
Zipline Downtime
December 8, 2025
Zipline will be unavailable while we make some updates to the e-IRB system the evening of December 8, 2025, from 5:30 pm to 8:30 pm Pacific Time. Please remember to save your work and log off before this time.
Dissolution of Committee B and Department Re-Assignment
This update includes the automatic re-assignment of departments currently assigned to team B. Refer to the newsletter item for more details.
Your new team assignment is listed on the Zipline dashboard:
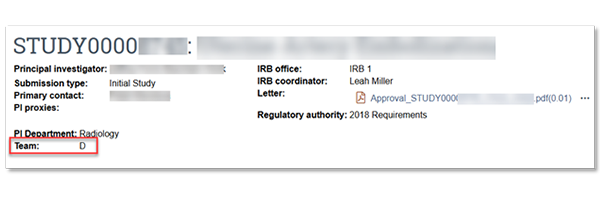
If you have any questions, please contact hsdinfo@uw.edu.
Revised Data Security Requirements
Reminder
In our July 1, 2025 newsletter we announced that HSD made significant changes to our GUIDANCE Data Security Requirements. This guidance applies to all research where the UW is the IRB of record, including non-UW sites relying on the UW IRB.
Compliance Deadlines
- New Studies: Were expected to comply as of the updated guidance effective date (July 1, 2025).
- Existing Studies: Studies approved prior to July 1st must be brought into compliance with the new requirements by January 1, 2026. Researchers are asked to assess whether their study’s data security level has changed and submit a modification in Zipline if needed.
Please review the updated guidance and contact hsdinfo@uw.edu if you have any questions.
Upcoming Webinars on OnCore Reporting for Diversity in Clinical Trials
Reminder
Thank you to everyone who joined the November 3rd informational session on OnCore reporting of enrollment data for studies subject to the UW Diversity in Clinical Trials (DCT) Policy. If you missed it, there are two remaining webinars:
- Register for December 11, 2025– 12:00-1:00 PM PT
- Register for January 14, 2026– 9:00-10:00 AM PT
These sessions will provide an overview of:
- The DCT Minimum Footprint requirements
- How to access and navigate OnCore
- Submitting new protocols for OnCore intake
- DCT protocol staff training and resources
Who should attend?
This training is intended for study teams preparing new clinical trials that will be submitted to HSD on or after January 1, 2026, and are subject to the UW DCT policy. If your trial already reports into OnCore for other reasons (e.g., oncology studies or those with UW Medicine billable activities), no additional reporting or training is required.
Register now to ensure your team is ready for these changes.
Longer Turnaround Times for External Authorizations for Clinical Trials
Plan Ahead
Beginning January 1, 2026, HSD anticipates longer turnaround times for authorization requests to rely on a non-UW IRB for clinical trials subject to the new Diversity in Clinical Trials policy. This is due to the additional time required to review the study’s diversity plan. As this process is new for both researchers and HSD, it is difficult to know exactly how much time this will add to the review. It will depend on the quality and completeness of the plan submitted and study team responsiveness to clarification requests. We anticipate that assessment of a diversity plan could extend HSD’s review time by several days to a week or more.
How can you ensure a shorter review time?
- Use the most recent version of the REQUEST External IRB Review form.
- Submit to HSD earlier in your process than you normally would. HSD can perform its authorization with draft versions of consent materials, and without a fully executed contract or negotiated budget. If you are waiting on minor administrative items such as a Worktag for billing, you may submit your application without that item. HSD will begin its review of the diversity plan, after which it will return the application to you with a request for any missing information.
- Ensure the Diversity in Clinical Trials policy applies to the research and that you include a complete diversity plan. Applications with missing or incomplete supplements will be immediately returned to the study team and will not be processed until complete.
- Respond promptly to clarification requests. Make sure that individuals who are responsible for the content of the diversity plan (e.g., PIs, Co-investigators, or sponsors) are available to answer questions.
Additional Recommendations
- Schedule Site Initiation Visits or other important study meetings with more lead in time than you typically would.
- Communicate to sponsors or others that this review is necessary under WA State law.
- Incorporate the final diversity plan into your application to the reviewing IRB.
Please contact hsdrely@uw.edu if you have any questions.