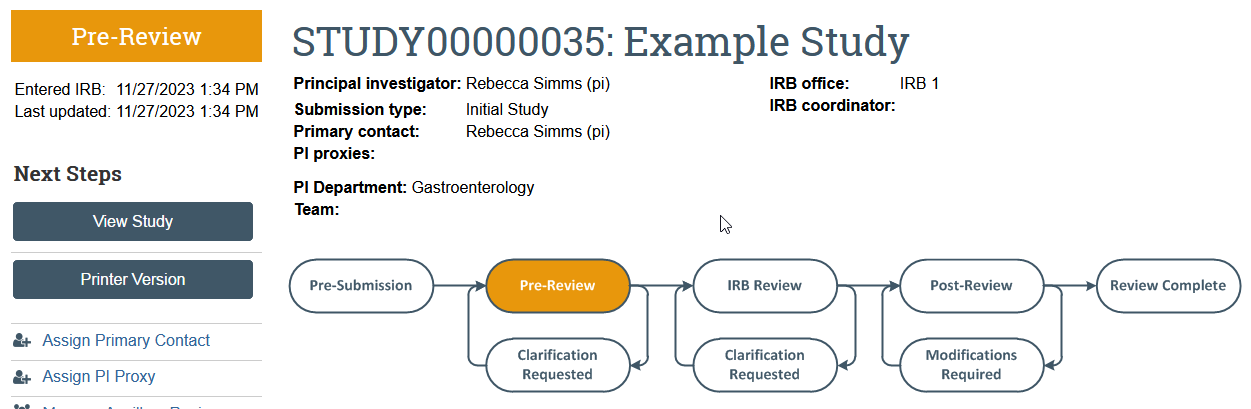
Apply for Review
Table of Contents
Before You Begin
Do you need to submit an IRB application?
Depending on the particulars of your project, you may not need to submit an IRB application at all! For more information:
Which IRB should review?
Applications can follow three basic review paths in Zipline. The paths depend on which IRB should conduct the review and whether the UW IRB should review on behalf of another institution.
These questions should be considered before the application is started and may require consultation with HSD. Contact hsdrely@uw.edu with any questions.
For more information:
Review Paths
Option 1: UW Reviewing for UW Only
This pathway is most appropriate for research that should be reviewed by UW and either:
- Does not involve any other institutions; or
- The UW should not review for other involved institutions. For example, the UW IRB does not generally review for the VA.
Option 2: UW Reviewing for Other Institutions
This pathway is most appropriate for research involving multiple institutions where the UW should review the activities of at least one other involved institution. In this pathway, the study approval is not approval for all sites. Instead, the other institution’s involvement is reviewed separately, and a separate site approval will be issued once that institution’s involvement is approved.
HSD must agree to review on behalf of the other institution and put reliance agreements in place.
Examples:
- UW is the primary recipient of a federal award for a multi-site NIH grant
- Working with local collaborators such as Seattle Children’s or the Hutch, and UW is the primary recipient of a federal award
- International studies where UW is the primary recipient of a federal award
For more information:
- UW IRB as a Single IRB
- Consult HSD’s Reliance Team at hsdrely@uw.edu
Option 3: Non-UW IRB Reviewing
This pathway is most appropriate for UW research that requires IRB review, but the UW IRB should not be conducting the review. Most of the time, studies that should be reviewed by an external IRB involve other institutions or industry funding.
HSD must agree in advance to cede IRB review to that IRB and any needed reliance agreements between institutions must be put in place.
For more information:
- Reliance on a Non-UW IRB
- Contact HSD’s Reliance Team at hsdrely@uw.edu with questions
Steps for Submitting
Overview
All applications must be submitted via Zipline, UW’s eIRB system. Zipline is a document management system where you upload your application form and other study documents and fill out a series of electronic SmartForms. For definitions of Zipline terms:
The order of the application steps is flexible. You may find it better to create the study record in Zipline and then complete the Word-based IRB application form, especially if you plan to be a PI proxy for a study.
Step 1: Set Up Zipline Access (One Time Only)
Everyone who needs access to the Zipline application must complete a one time self-registration process in Zipline before they can be added to the application. Each person must be added to the application before they are able to access it. This includes:
- The principal investigator
- Any study team members who will need to view or edit the application
- Faculty advisors (for student and medical resident applications)
A UW NetID is required to register. For more information:
Step 2: Complete Word-Based Application Form
All applications must include one of the following application forms. Use the most recent version of the form, available on the HSD website and in Zipline. Upload documents in Microsoft Word format. UW offers Microsoft Office to UW students, faculty, and staff at no charge.
- IRB Protocol Form: Standard application form used to request review from HSD or the UW IRB.
- IRB Protocol Form, No Contact: Shortened version of the standard IRB application form that may be used for studies that do not involve interaction of any kind (in person, email, internet, social media, etc.) with subjects.
- External IRB Review Request Form: Use this form to request authorization to obtain IRB review from a non-UW (“external”) IRB instead of the UW IRB.
IRB Protocol Tips
- The IRB Protocol has sections based on topic. The first time you complete the form, scan the questions so that you know what is coming and don’t duplicate information.
- Use the instructions and examples embedded within the form to help answer questions you may have about completing various sections.
- If you think that your research may qualify for a determination that it is exempt or is not human subjects research, only answer the questions labelled [DETERMINATION] on the IRB Protocol. If HSD staff determine that additional information is needed, they will ask you to complete additional questions.
- Reference other documents in the application, such as grant applications or study protocols, if they provide the needed information and are attached to the electronic application.
- The IRB Protocol is a living document that gets updated to provide complete and accurate information about your approved research activities. Use tracked changes to make changes requested by the IRB and to modify your study.
Step 3: Prepare Other Documents
In addition to the HSD application form, we recommend preparing all other study documents before creating your application in Zipline. The study documents should be uploaded in Microsoft Word format whenever possible.
Requests to Use a Non-UW IRB:
Review Section 5: Required Attachments in the Request External IRB Review form for a list of documents that should be included with an application requesting review by a non-UW IRB.
UW Reviewed Studies:
You will need:
- IRB Protocol Form (Standard or No Contact)- Microsoft Word format only (Review Step 2)
- Grants, contracts, or other funding attachments (if applicable).
- Study drug or device attachments, such as Investigator Brochures, IND information, device instructions (if applicable).
- Consent forms, assent forms, and parental permission forms (if applicable) – Microsoft Word format only.
- Any recruitment materials, including all material to be seen or heard by subjects. HSD encourages researchers to upload descriptions of most recruitment and screening materials instead of the materials themselves. The goal is to provide the researchers with the flexibility to change some information on the materials without submitting a modification for IRB approval of the changes.
- Curriculum vitae (CV) or biosketch for the UW/Zipline principal investigator.
- IRB 101 Training certificate for student and medical resident principal investigators.
- Any other study documents, such as data collection forms; study instruments, tools, or surveys; or application supplements.
Step 4: Create Study Record in Zipline
Once your documents are ready, login to Zipline to create a new study record and upload the study documents.
The basic process for creating a new study is the same for all applications. After creating the study, you or other members of the study team who are listed on the Study Team Members page can continue to edit the application as needed until it is ready to be submitted to HSD for review. For detailed steps with screenshots, review submit initial application steps.
External (Non-UW) IRB Review: Tips for Completing SmartForms
Review Section 5: Required Attachments in the Request External IRB Review form for a list of documents that should be included with an application requesting review by a non-UW IRB.
UW IRB Review: Tips for Completing SmartForms
| SmartForm Name and Attachments | SmartForm Tips |
|---|---|
| Basic Information SmartForm Page
Attachments:
|
Only upload the IRB Protocol in Microsoft Word format. No other documents should be uploaded in this question. |
| Study Funding Sources SmartForm Page
Attachments:
|
For funding administered through UW:
|
| Local Study Team Members SmartForm Page
No Attachments |
Can’t find someone on the list? Make sure that they’re registered in Zipline.
Add everyone who needs access to the application, including anyone who should be designated as a PI proxy. People must be listed on this page in order for the PI to be able to designate them as a proxy. Students/medical residents- Do not list your faculty advisor. Adding your faculty advisor on this page prevents them from completing faculty advisor review of your application. |
| Drugs SmartForm Page
Attachments:
|
|
| Devices SmartForm Page
Attachments:
|
|
| Study Related Documents Page (Only available for multi-site or collaborative studies in which the UW is reviewing for another institution)
Attachments:
|
Only attach documents that apply to the overall study and are not specific to the UW site, such as generic study consent templates that will be adapted for each site. Documents specific to the UW site should be attached on the Local Site Documents Page. |
| Local Site Documents Page
Attachments:
|
Consent Forms:
Recruitment Materials:
CVs, Training Certificates, and Other Documents:
|
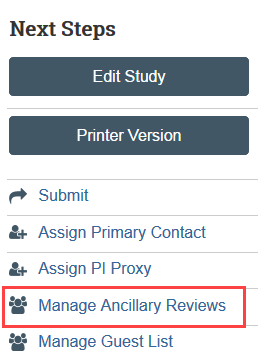
Step 5: Add Faculty Advisor Review (Students and Medical Residents Only)
The Manage Ancillary Review activity is used to obtain and document faculty advisor review when a student or medical resident is the principal investigator (PI) for the IRB application. Do not add the faculty advisor to the study team on the Zipline application before the faculty advisor has submitted the faculty advisor review. The system will not allow a member of the study team to submit an ancillary review for the study.
The faculty advisor review may occur concurrently with HSD or IRB review of an application. However, final IRB approval will not be issued until the required faculty advisor review is completed.
Your faculty advisor will receive an email notification when the application is submitted to HSD. For more information:
- FAQ: What are the additional requirements for student and medical resident researchers?
- Add Faculty Advisor Review steps
Step 6: Review Application for Completeness
Checking the application for errors and omissions helps you to include all the relevant information, which is essential for receiving a timely review of your study. Automatic system error checking identifies any omitted answers to questions that are required for every application. A red asterisk (*) indicates required questions.
HSD also recommends looking over the forms to see what you may have missed, especially:
- Questions that are relevant to your study but are not required for all studies (no asterisks)
- Documents that should be attached
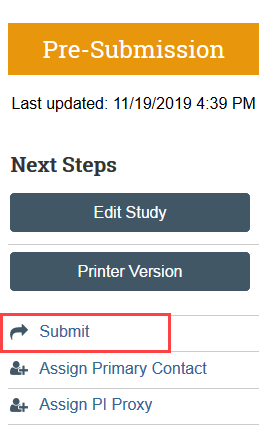
Step 7: Principal Investigator (PI) or PI Proxy Must Submit
The Submit activity must be completed once the application is ready to go to HSD. This activity:
- Must be completed by the PI or the PI Proxy
- Changes the application state from Pre-Submission to Pre-Review
- Sends the item to HSD for review
- Removes the study team’s ability to make edits while the study is in review unless HSD requests more information or a change to the study
For more information:
What to Expect After Submitting
An HSD staff member will be assigned to your application as the IRB coordinator. The PI, any PI proxies, and the primary contact will receive email notifications:
- If HSD staff need additional information or changes to complete the review
- To inform you about the outcome of the review, including a formal determination letter with more information
You can check the application status in the study workspace in Zipline at any time.