Renew Your IRB Approval
Table of Contents
Before You Begin
Does your study require continuing review?
| Review Type | Continuing Review Requirements |
|---|---|
| Determinations (Exempt, Not Human Subjects Research, Not Research, UW Not Engaged) | Continuing review not required. Do not submit a request to renew. |
| External (Non-UW) IRB Review | Apply for continuing review with the reviewing IRB if required for the study. You do not need to submit documentation of renewed IRB approval to HSD. Review the External IRB Checklist for UW Researchers for more information. |
| UW IRB Approved Studies | Not all studies that receive UW IRB approval require continuing review. If your study requires continuing review:
If you have completed all recruitment, study procedures, and analysis of identifiable data, you should close your IRB application instead. |
| Multi-institutional UW IRB Approved Studies | Continuing review is conducted at the study level. Any information about other institutions relying on the UW for review should be reported with the overall study continuing review. Review Multi-site Continuing Review and Closure for detailed steps. |
How early should you apply for continuing review?
Many applications for IRB renewal must be reviewed by the convened IRB instead of by an expedited reviewer, so make sure to give the IRB plenty of time to review your application! We recommend submitting your continuing review application at least 45 days before approval expires to avoid a lapse in approval.
What happens if IRB approval expires?
All human subjects research activities must stop. This includes recruitment, advertisement, screening, enrollment, consent, interventions, interactions, and collection or analysis of private identifiable information. Also, funding related to those activities or that was released because of a DOHR determination cannot be spent.
Exceptions: If any of the following exceptions apply, immediately report this to the UW IRB including information about the continued research activities and the rationale for continuing them:
- Continuation of activities necessary to eliminate apparent, immediate hazards to subjects.
- The research intervention (if any) may be of direct benefit to individual subjects.
- Withholding the research intervention (if any) may increase risks to subjects.
Submit a renewal or close your application as soon as possible. If a renewal or closure is not received and IRB approval has been lapsed for 90 days HSD may administratively close the study.
In some circumstances, HSD may refuse to review additional submissions from the researcher until a status report is received and approved or the application closed. Lapsed IRB approval may be considered continuing noncompliance, and the study may be “terminated” by the IRB.
Review section 5.4 of SOP IRB Review for more information.
Steps to Renew Your IRB Approval
Step 1: Complete the Status Report Form
To request renewal of IRB approval, complete the Status Report form.
To request renewal of a Delayed Onset Human Research (DOHR) determination, complete the APPLICATION Determination, Delayed Onset Human Research and follow the instructions on the form.
Step 2: Prepare Any Supporting Documents for Upload (If Needed)
Most continuing review applications only require the status report form, but you have the option to upload other documents related to the continuing review such as sponsor progress reports or radiation safety renewals.
Any Data and Safety Monitoring Board (DSMB) reports should be submitted separately using the reporting process.
Step 3: Complete the Continuing Review in Zipline
- Navigate to the study you need to renew and click Renew or Close under Next Steps
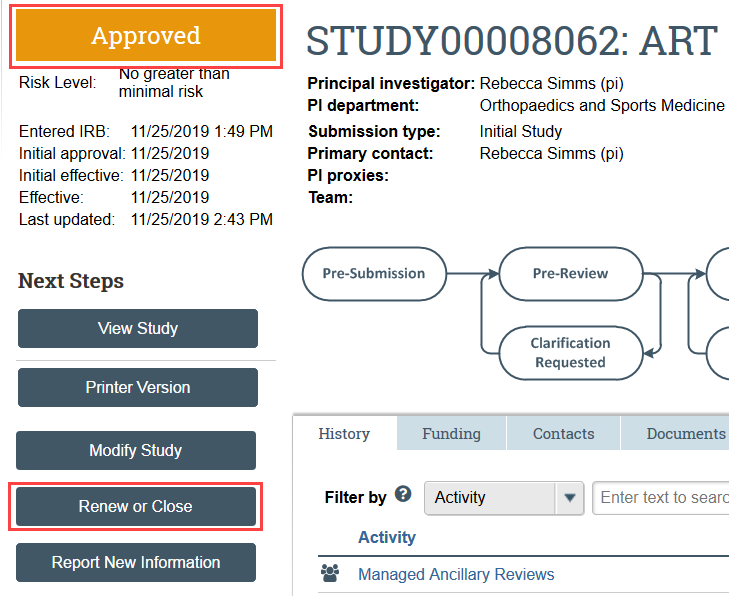
- Complete the forms and upload the status report form and any supporting documents
Common Mistakes:
- Missing explanations for items or events from the past year. Make sure that you accurately checked all applicable items and provided a summary explanation for each item you did not check.
- Checking the incorrect research milestones. Many researchers believe that IRB approval must be renewed if any identifiable private information or specimens will be retained. However, the IRB application may be closed if the data analysis described in the IRB application has been completed and if retention of the identifiable data is consistent with information in the IRB application.
- Not accurately reporting subject numbers for multi-site studies. Make sure to distinguish between the number of subjects for the UW’s site versus the number of subjects across the entire multi-site study.
For step by step instructions:
Step 4: The PI or a PI Proxy must submit
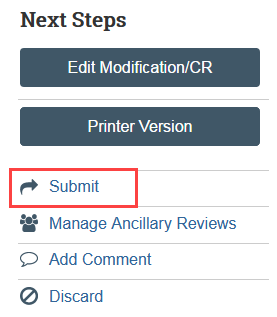
The Submit activity must be completed by the PI or a PI proxy once the continuing review is ready to go to HSD.
- Select Submit in the continuing review workspace and provide required verifications
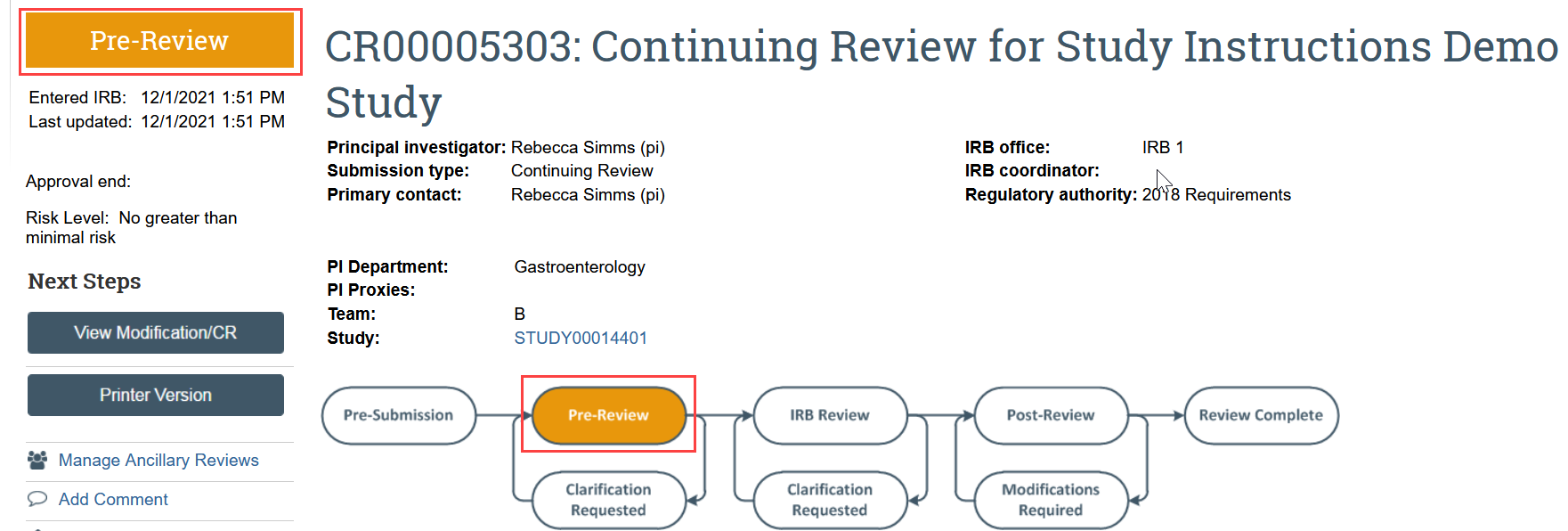
What to Expect After Submitting
The continuing review transitions to Pre-Review state and is now in HSD’s queue for review.
After the IRB has reviewed the application, the PI, any PI proxies, and primary contact will receive a notification that:
- The IRB has approved the renewal; OR
- The IRB requires more information or a change before approving the renewal
Visit Respond to HSD for more information on submitting responses.
After Continuing Review Approval
After the continuing review is approved, the parent study will be updated. The new expiration date is published to the parent study. The PI, PI proxy, and primary contact also receive a notification containing the Continuing Review approval letter.
