May 15, 2025
May 15, 2025 SAGE Suite Release Notes
The following email was sent to the SAGE and MRAM mailing lists on Thursday, 5/15/25.
The following features were released to SAGE on the evening of May 15. If you have any questions, please reach out to us at sagehelp@uw.edu.
Goal: Add Clarity and Reduce Returns for Campus
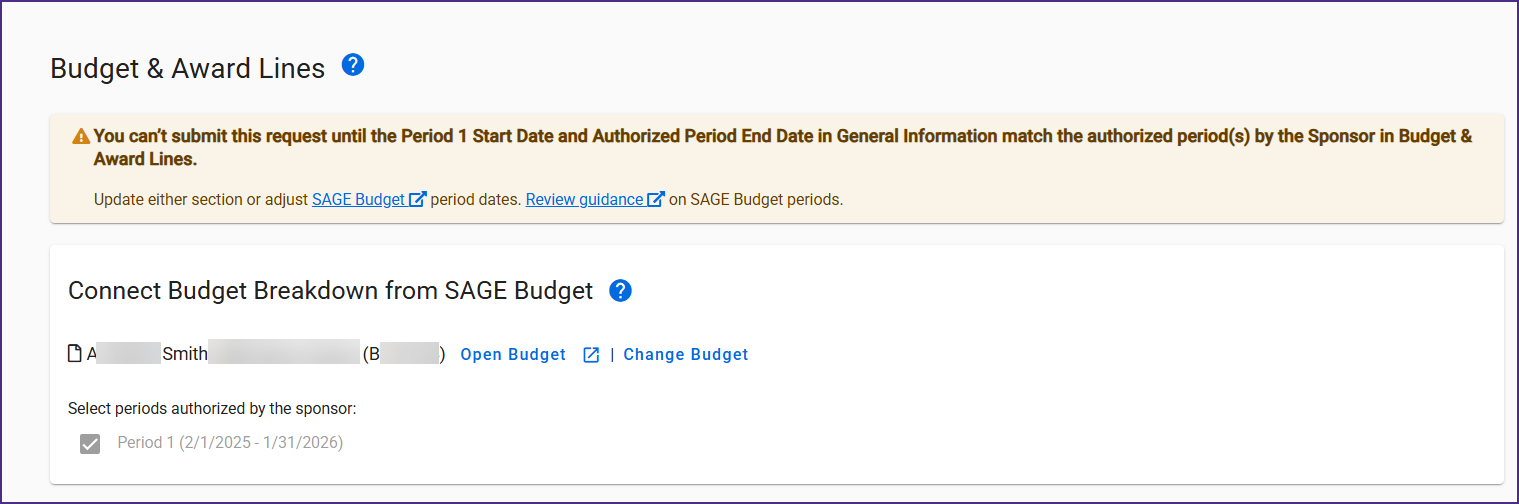
New Award Setup Request Validations
In order to reduce returns of Award Setup Requests (ASRs), new validations have been implemented for ASRs:
- Start and End Dates: When connecting a budget to an ASR, the Period 1 Start Date and Authorized Period End Date in the General Information section must match the sponsor’s authorized period(s) in the Budget & Award Lines section. If the dates do not match, users will see a warning message and be unable to submit the ASR for routing.
- Sponsored Program Activity (SPA) Type: When connecting a budget to an ASR, the SPA type in the ASR’s General Information section must match the connected budget’s Primary Worksheet’s SPA type. If they do not match, users will see a warning message and be unable to submit the ASR for routing.
Note: If non-primary worksheets show a different SPA type from the ASR’s General Information section, users will see a warning but submission will still be allowed.

Award Setup Request Start & End Date Label Changes
To provide added clarity for campus, the following label changes have been made on the Award Setup Request:
- Current Period Start Date has been changed to Period 1 Start Date.
- Current Period End Date has been changed to Authorized Period End Date.
Goal: Address High-Priority Maintenance Needs
eGC1 Compliance Question Updates
In preparation for the upcoming United States Government Policy for Oversight of Dual Use Research of Concern (DURC) and Pathogens with Enhanced Pandemic Potential (PEPP), updated questions and guidance have been added to the eGC1 Non-Fiscal Compliance section. Review the Updates to the eGC1 Non-Fiscal Compliance Section job aid.
All eGC1s in “Composing, “Withdrawn,” or “Returned” statuses at the time of the 5/15/2025 SAGE release will be required to re-answer the EH&S non-fiscal compliance questions. Any eGC1s in other statuses will not require updated answers.
The Environmental Health and Safety (EH&S) subsection now includes the following questions:
- Biohazards: When selected, the following new guidance is provided:
- Biological Use Authorization (BUA) from the UW Institutional Biosafety Committee (IBC) is required for research involving biohazards, including recombinant or synthetic DNA/RNA. Some categories of research require BUA prior to initiation. For more information, including the IBC’s definition of a biohazard and submission dates for IBC review, please refer to Biological Research Approval | UW Environmental Health & Safety.
- The Select Agents and Toxins label has been updated to Risk Group 3 or 4 Microorganisms or Select Agents and Toxins. When selected, the following new guidance is provided:
- Prior to initiation, research with most Risk Group 3 and 4 microorganisms and all select agents and toxins are subject to review for potential Dual Use Research of Concern (DURC) and Pathogens of Enhanced Pandemic Potential (PEPP) by the UW Institutional Biosafety Committee (IBC) and the Institutional Review Entity (IRE) subcommittee. For a list of agents and toxins subject to the policy and information about assessing your research for DURC and PEPP, refer to Dual Use Research of Concern (DURC) and Pathogens with Enhanced Pandemic Potential (PEPP) | UW Environmental Health & Safety.For use of select agents and toxins, EH&S will work with the PI to obtain necessary federal clearances, approvals, and training. Documentation of due diligence is required for transfer of select toxins in any amount. For more information, refer to Select Agent Program | UW Environmental Health & Safety.
- The Diving label has been updated to SCUBA Diving.
- Chemical thresholds: When selected, the following new guidance is provided:
- Select if your research will require the use of highly toxic, highly unstable, highly water reactive, pyrophoric, flammable gas, explosive or materials of similar hazard in quantities in excess of this table. EH&S will contact you for planning assistance as fire department requirements may not allow these quantities in your space or may require building modifications.
- Radioactive materials: No changes.
SAGE-to-Workday Integration
Change in Grant Resource Worktag Mapping (Released 5/06/25): The grant resource worktag applied to awards with foreign sponsors (excluding subawards where the prime sponsor is federal) has been updated per GCA’s request via integration between SAGE and Workday. The resource worktags used for foreign sponsored awards are now either RS100078 Non-Governmental or RS100206 Non-Governmental Grant-in-Aid Resource.
Change in Object Code Mapping (Released 4/14/25): The SAGE to Workday integration for Award Setup Requests has been updated to map the SAGE Budget 08-05 sub-object code to the new Workday ledger account 40070: Fee-Based Tuition and Fees Charged to Grant.
Miscellaneous Maintenance
- Fix: An issue was fixed where the ad hoc approver “Approve Request” button was not always displaying on Modification Requests.
- Fix: OSP auto-assignments for Non-Award Agreements (NAAs) were corrected for federal sponsor types.
- The eGC1 Certify and Route page was updated to correct “Disbarment Statement” to “Debarment Statement.”
- Where duplicate awards exist in Workday, SAGE Awards was updated to align with active Workday awards.
Updated SAGE Documentation
As part of this release, the following SAGE documentation pages were updated: