Respond to HSD
Overview
During review of initial applications, modifications, continuing review reports, and reports of new information, HSD staff may contact you requesting more information and/or changes to the application. Three different scenarios require the study team to respond to HSD to move the review forward.
In all three scenarios:
- The PI, any PI proxies, and the study’s primary contact receive an email notification and the study appears in My Inbox.
- A member of the study team may need to edit the related documents and/or SmartForms based on HSD or the IRB’s request.
- The PI or a PI proxy must complete the Submit Response activity to push the application back to HSD before HSD may take additional action.
If you do not respond, your submission may be withdrawn after 6 months of non-response.
For more information on responding to HSD during relying site review, visit Submitting New Sites.
Scenarios
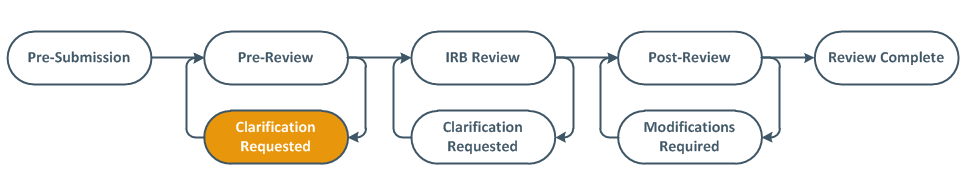
Clarification Requested- Most Situations
At any stage during the review process, the HSD or IRB reviewer may request clarifications to the application content. This moves the application to the Clarification Requested state.
- Clarification Requested (Committee Review) Only: The study team can only provide a written response and cannot edit the study. The study can be edited with any needed changes after review by the convened IRB.
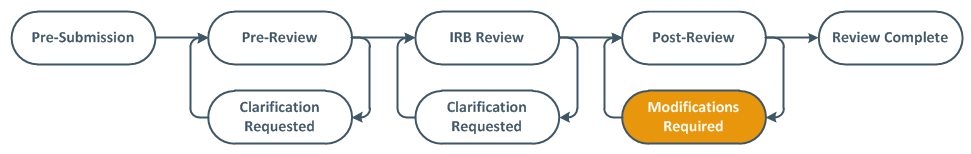
Modifications Required to Secure Approval (MRSA)- Usually Full Board Studies
The IRB may determine that the study requires changes or the IRB requires more information before the research can begin. The PI, PI proxies, and primary contact are sent an email notification.
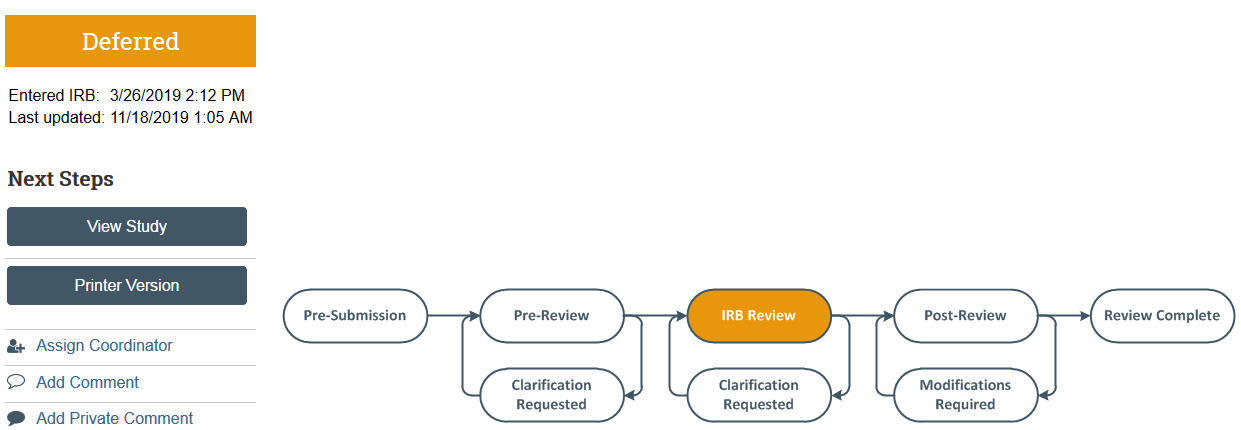
Deferral- Full Board Studies Only
When the convened IRB requests significant additional information in order to complete the review, the PI, PI proxies, and primary contact are sent an email notification and the application moves to Deferred state.
How to Respond to HSD
Step 1: Locate the clarification request or review letter
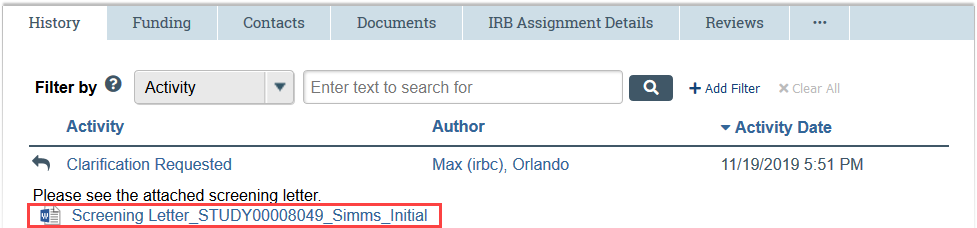
FOR CLARIFICATION REQUESTS:
- Locate the Clarification Requested activity in the History tab of the application workspace
- Review the text note and click to open any attachments
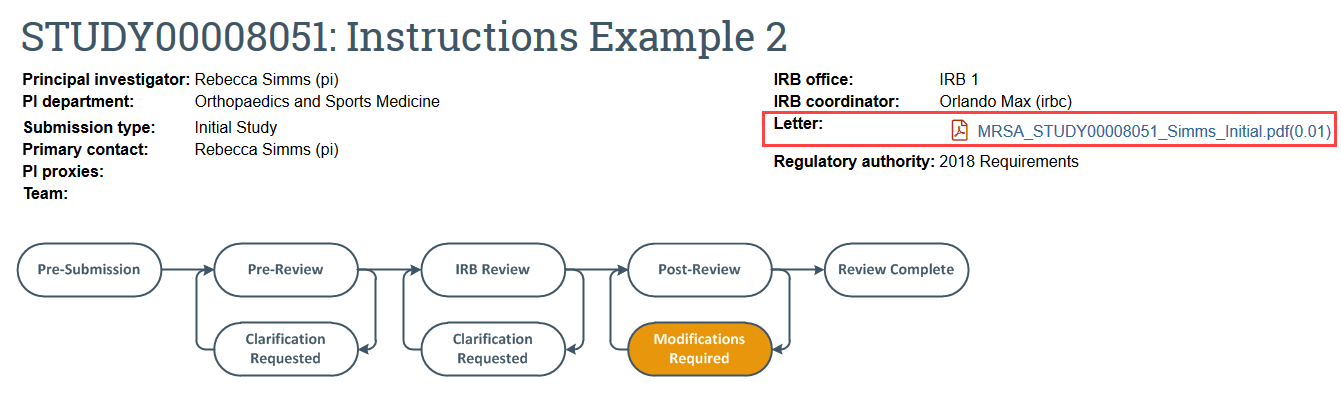
FOR MODIFICATIONS REQUIRED AND DEFERRAL:
- Click the letter link near the top of the study workspace for the formal letter
Step 2: Prepare your written response
FOR CLARIFICATION REQUESTS:
- If a review letter is attached, save a copy of the review letter and embed your responses in the body of the letter to facilitate review.
FOR MODIFICATIONS REQUIRED AND DEFERRAL:
- Write a point-by-point response letter by creating a new Word document, copying the IRB’s letter into the document, and responding to each point. Number your responses to match the points listed in the original letter to facilitate review.
Step 3: Prepare updated study documents (as needed)
- Make sure you’re updating the most recent version that HSD reviewed. There are 2 ways to pull the most recent version from Zipline:
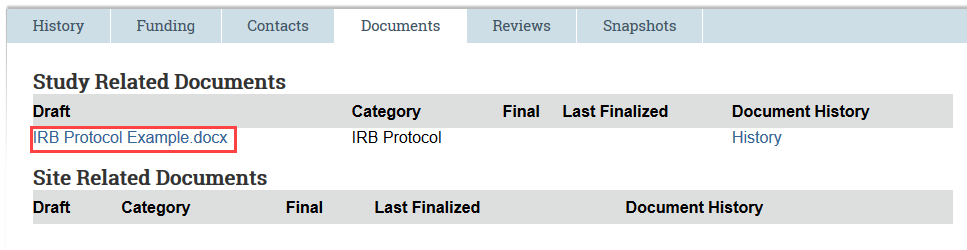
- Option 1: From the Documents Tab
- Navigate to the Documents tab and select the draft version of the document.
- Make edits and save the document to your computer. To aid in HSD’s review, make sure tracked changes is enabled.
- Option 1: From the Documents Tab
-
- Option 2: From the SmartForm
- Select Edit Study (or Modification/CR/RNI) and navigate to the page containing the document that you need to update.
- Option 2: From the SmartForm
-
-
- Click the link for the document to open the most recent draft version that HSD reviewed.
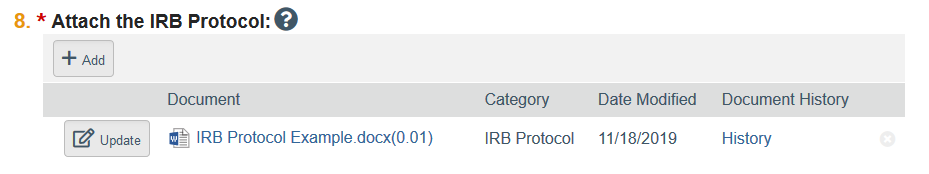
- Make edits and save the document to your computer. To aid in HSD’s review, make sure tracked changes is enabled.
-
Step 4: Make Zipline edits and upload revised documents (as needed)
- Select Edit Study (or Modification/CR/RNI) and use the lefthand navigator or the Continue button to progress through the forms making changes as needed

To Upload Revised Study Documents:
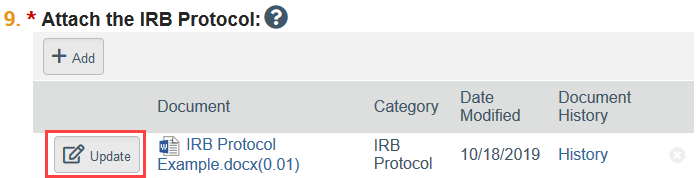
- Select Update by the revised document in the SmartForm. This allows you to replace the old document with the revised version which helps the IRB to see the changes between versions of the documents during the review process.
- NOTE: Select Add if you are uploading a completely new document, and click the X button to remove a document from the study
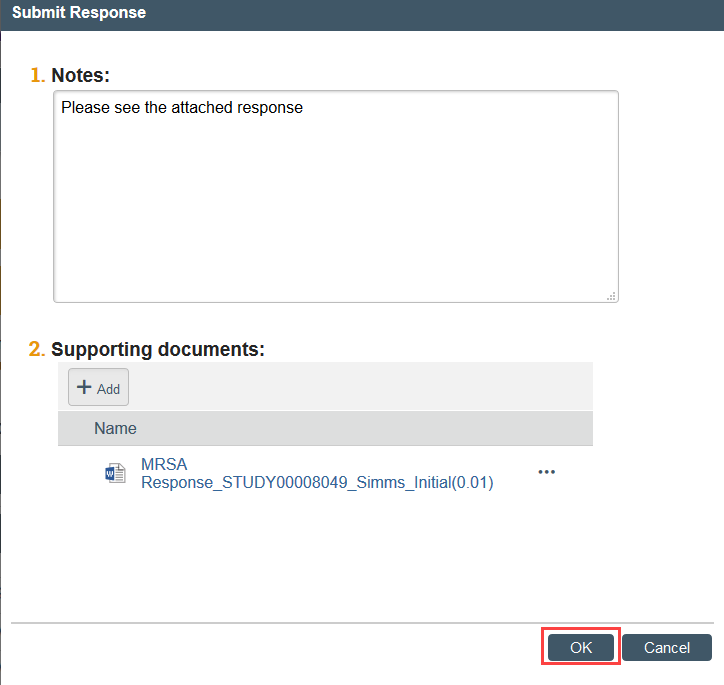
Step 5: PI or PI Proxy must Submit Response
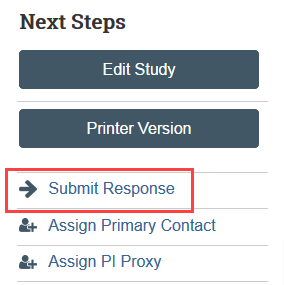
- Enter any notes and/or attach your written response and click OK