Human Subjects Division
Managing Multi-institutional Studies
 Print
Print
When a multi-institutional study is initially approved, the IRB first approves the overall study protocol and the involvement of the UW. After that, the IRB will review site specific information for each relying institution and approve each site separately. See Submitting New Sites for detailed instructions on this process.
Actions that may be needed for multi-site studies:
- Submitting New Sites
- Multi-site Study Continuing Review or Closure
- Site Closure
- Multi-site Study Modifications
Multi-site Continuing Review or Closure
Continuing review is conducted at the study level. Any information about other institutions relying on the UW for review should be reported with the overall study continuing review. The last day of approval for a site is the same as the last day of approval for the study overall.
The continuing review report is used both for study renewal and study closure. When you indicate on the continuing review SmartForm that the first four research milestones have been met for the entire study, Zipline prompts you to acknowledge that the study will be closed. These milestones indicate that all enrollment, interventions, and analysis of subjects’ private identifiable information are complete at all sites.
The continuing review report has two parts:
- The Continuing Review SmartForm page in Zipline
- The Status Report form that you complete and then attach to the SmartForm
How to Submit Multi-site Continuing Review
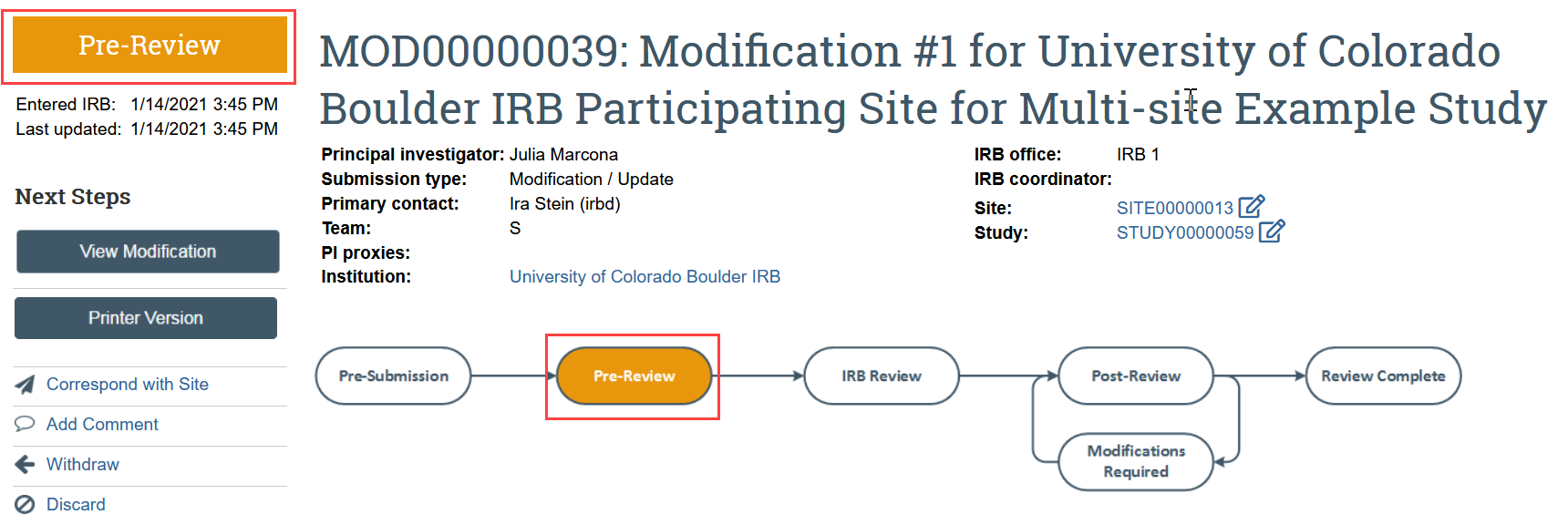
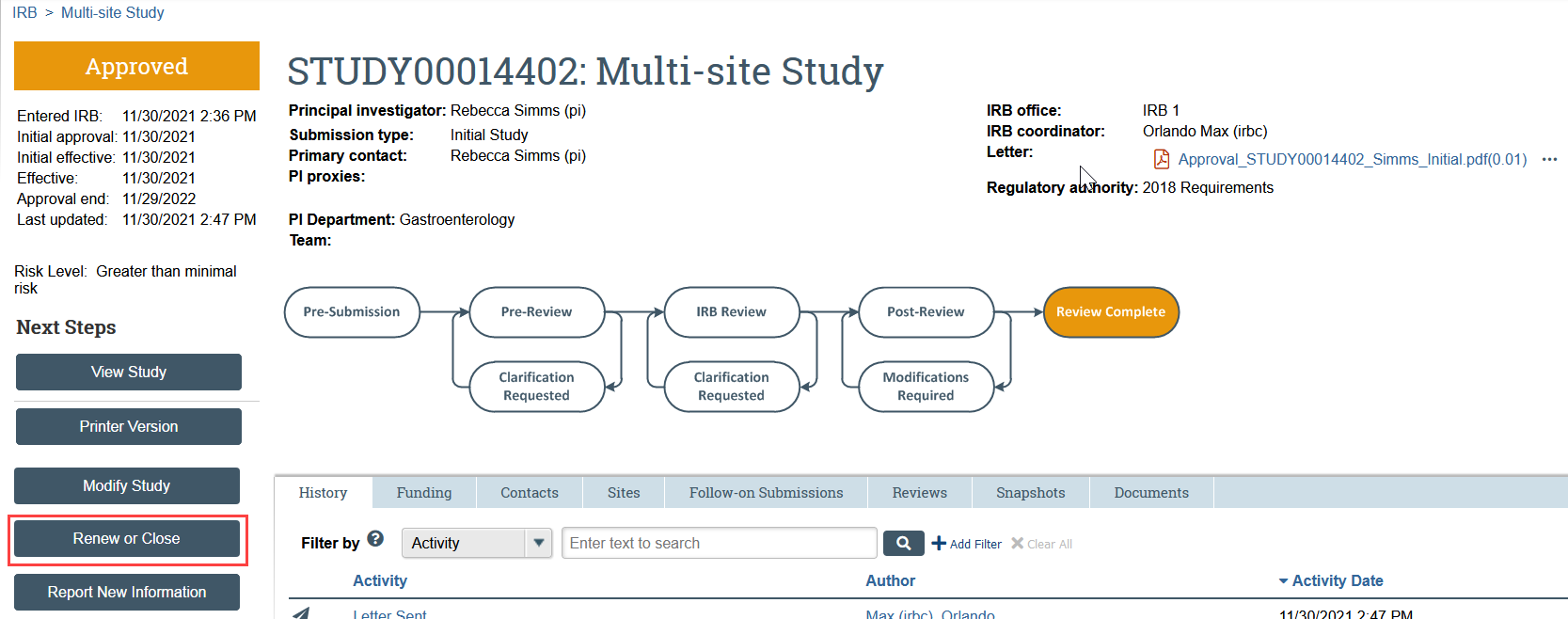
- Click Renew or Close in the study workspace
- The study must be Approved, Lapsed, or Suspended for this action to be available.
- Indicate that the purpose of the submission is continuing review and click Continue
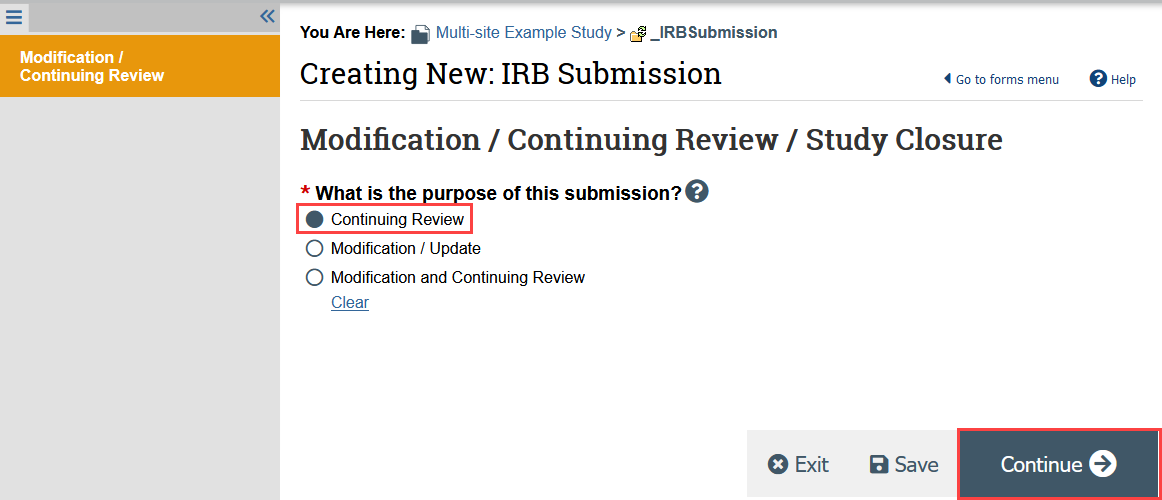
Your responses should include information for all participating sites.
- The total enrollment for Psites, or participating sites, with completed reports is a system generated function that UW is not currently using. You can ignore this.
- You will be prompted to acknowledge that the study will be closed if the first 4 research milestones are selected.

Complete and upload the Status Report form. If your study requires radiation safety approval at UW, you should also attach your radiation safety renewal here.
- Click the link to open the template
- Complete the template and save it to your computer
- Attach the template to the SmartForm, either using drag and drop, or by clicking Add
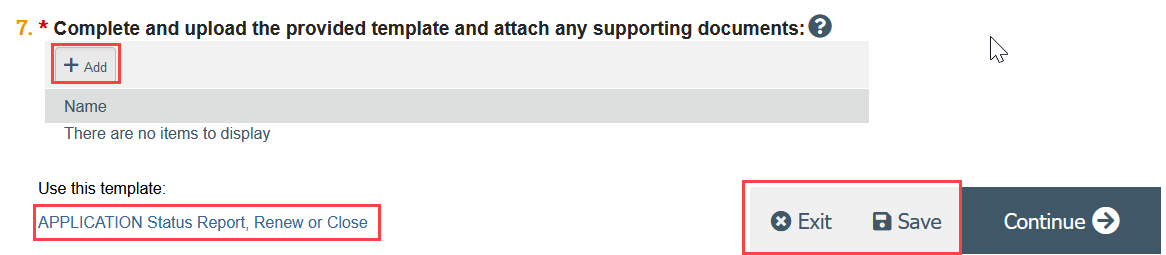
- Click Save and Exit to leave the SmartForm and return to the submission workspace
- Click Submit and complete required verifications
The continuing review transitions to Pre-Review state and is now in HSD’s queue for review.
After the IRB has reviewed the application, the PI, any PI proxies, and primary contact will receive a notification that:
- The IRB has approved the renewal or closure; OR
- The IRB requires more information or a change before approving the renewal or closure
Review Respond to HSD for more information on submitting responses.
After Continuing Review Approval
After the continuing review is approved, the parent study will be updated. For closure requests, the parent study and all participating sites are closed. For renewal requests, the new expiration date is published to the parent study and applies to all participating sites. The PI, PI proxy, and primary contact also receive a notification containing the Continuing Review or Closure approval letter.
Site Closure
If the involvement of a relying institution has ended, submit a site closure to the IRB. This closes the SITE record while leaving the parent STUDY record open. To close the study, follow the Multi-site Continuing Review or Closure instructions.
How to Submit a Site Closure
- In the parent study for the site, locate and download the currently approved SUPPLEMENT: Multi-site or Collaborative Research in the Documents tab
- Update the SUPPLEMENT: Multi-site or Collaborative Research to remove the information for the site that you wish to close
- Save the revised SUPPLEMENT to your computer
- In the parent study for the site, click Modify Study to initiate a study modification
- Indicate that the purpose of the submission is a modification/update and the modification scope is “Other parts of the study” and click Continue
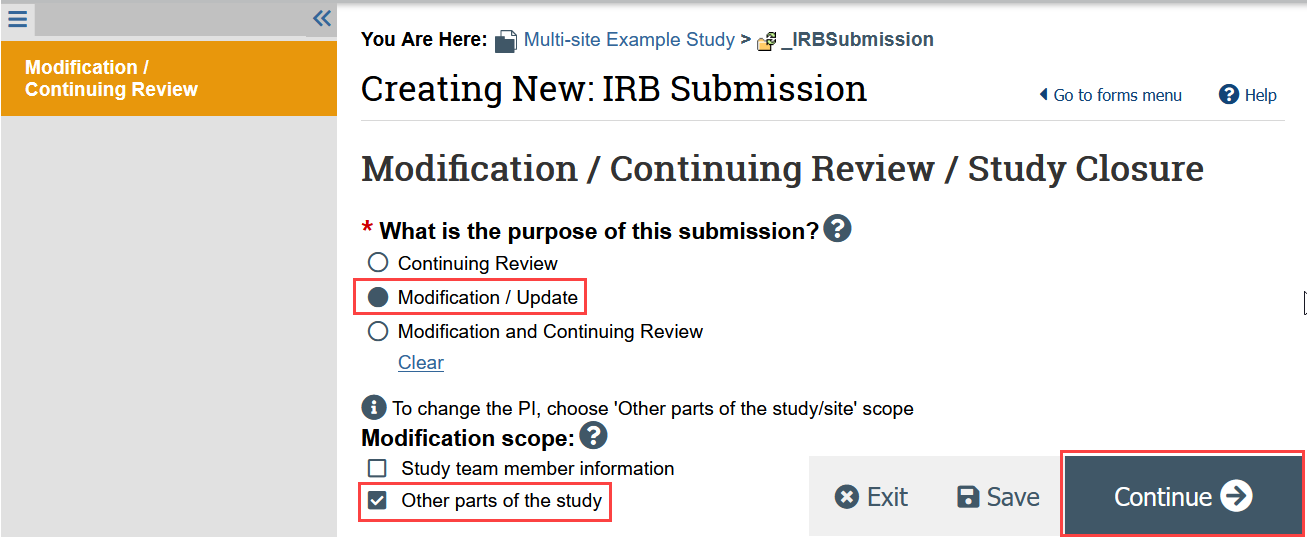
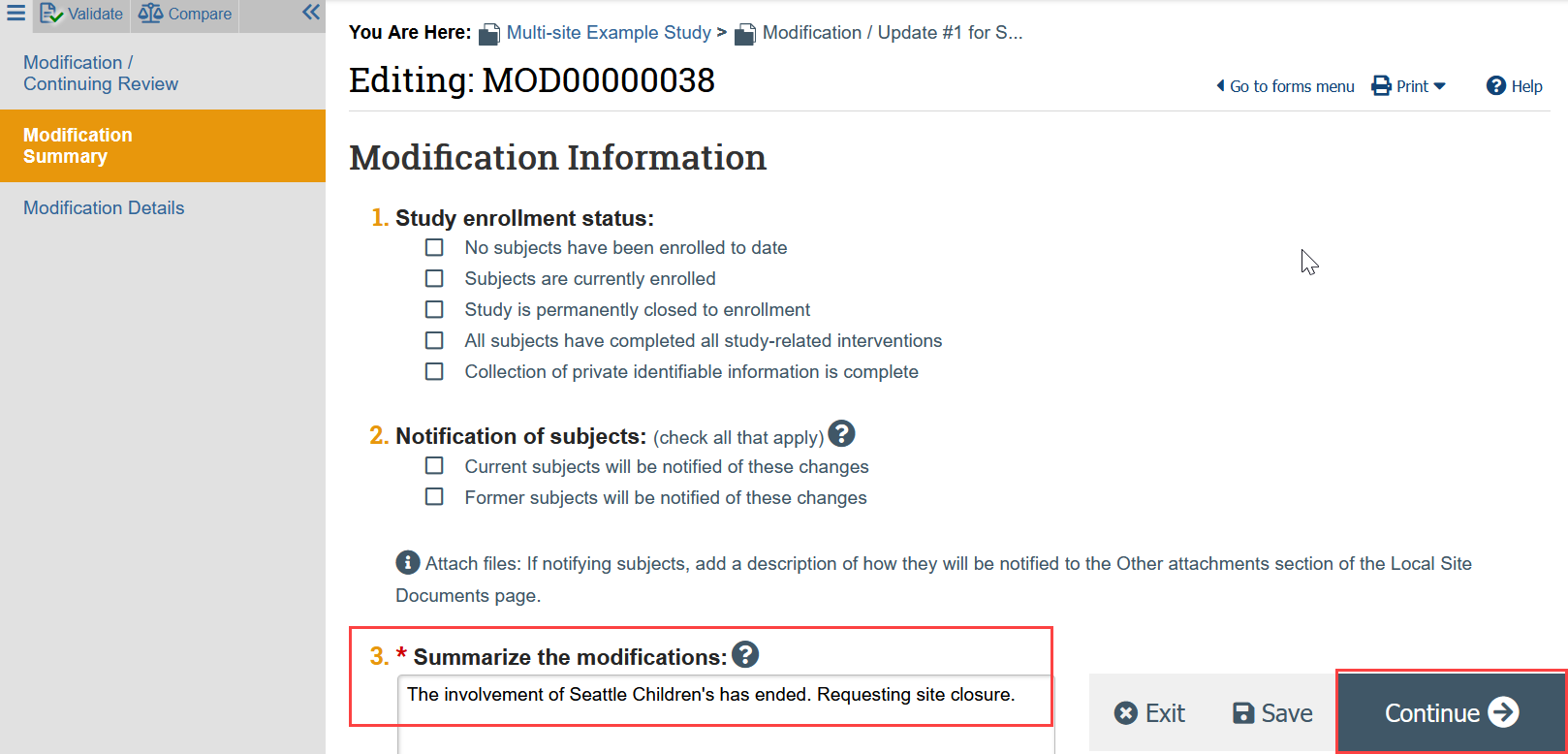
- On the Modification Summary page, indicate that the involvement of a relying institution has ended and the site should be closed, and click Continue
- In the Zipline application, use the left hand navigator to go to the Study Related Documents page and locate the SUPPLEMENT: Multi-site and Collaborative Research
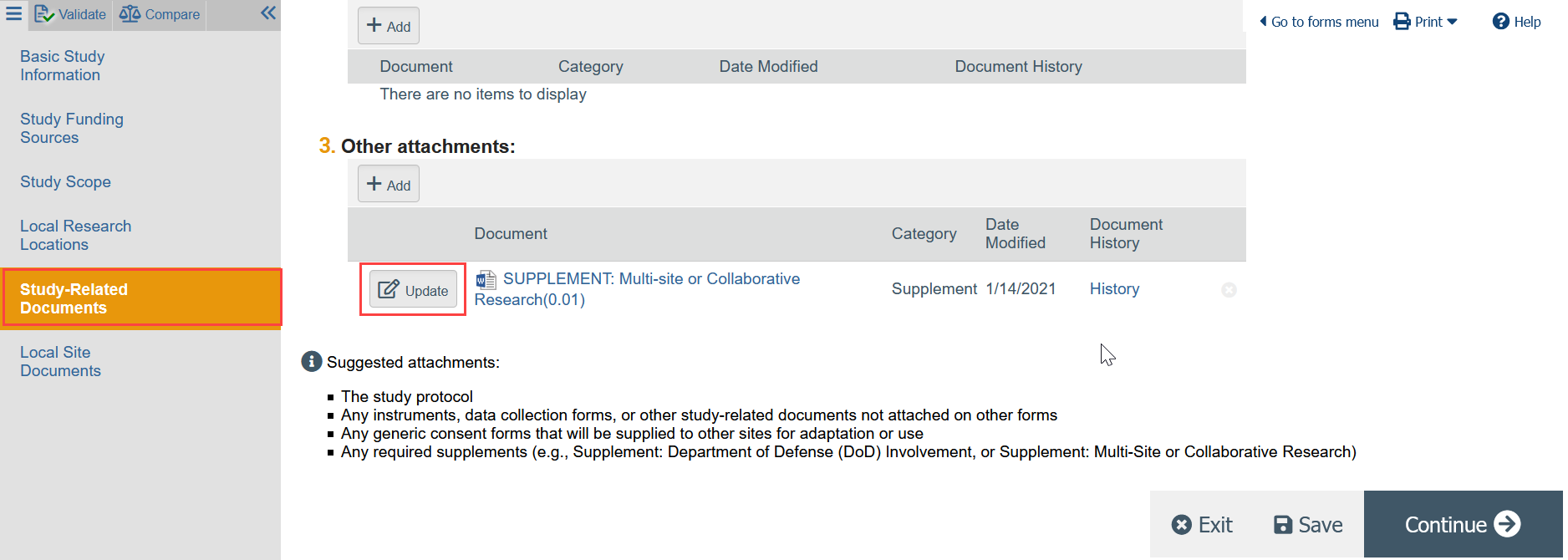
- Click UPDATE next to the SUPPLEMENT, select the revised version from your computer, and click OK
- If desired, you can make additional changes to your study application
- Save and exit the SmartForm
- In the modification workspace, click Submit to send the modification requesting site closure to HSD
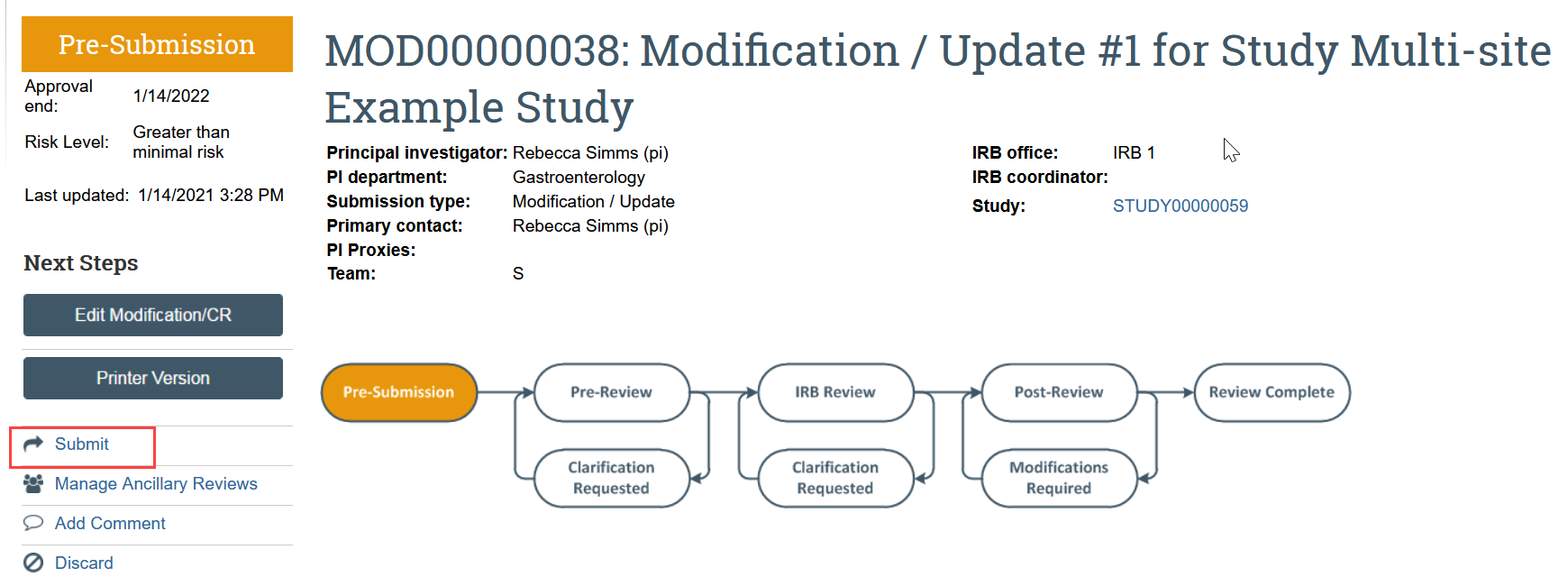
Once HSD has approved the modification updating the SUPPLEMENT: Multi-site and Collaborative Research, HSD staff will close the SITE record in Zipline.
Multi-site Modifications
Depending on the change requested, multi-site studies may need to be modified at the study level and/or the participating site level. Because the type(s) of modifications will depend on the study and how it is set up in Zipline, it can be tricky to determine if a site modification is required.
To initiate any changes to a multi-site study or site:
First, consider whether the changes apply to the overall study only, to a specific site only or impact both the overall study and any sites. If you are uncertain, email hsdrely@uw.edu.
Study-level modifications. If the changes affect the overall study or both the study and sites, submit a study level modification. HSD strongly recommends that you wait to submit the site level modifications until the study level modification has been reviewed. HSD staff will automatically evaluate if a site modification is also required as part of the review process and will provide instructions about when and what to submit for a site level modification. If any related site modifications are needed, HSD staff will instruct you to create them. The PI or PI proxy must then submit the site modification before HSD staff can complete the review.
Site-level modifications. If the changes affect only a site, submit a site modification following these instructions:
How to Submit Site Modification
- In the SITE workspace, click Create Site Modification
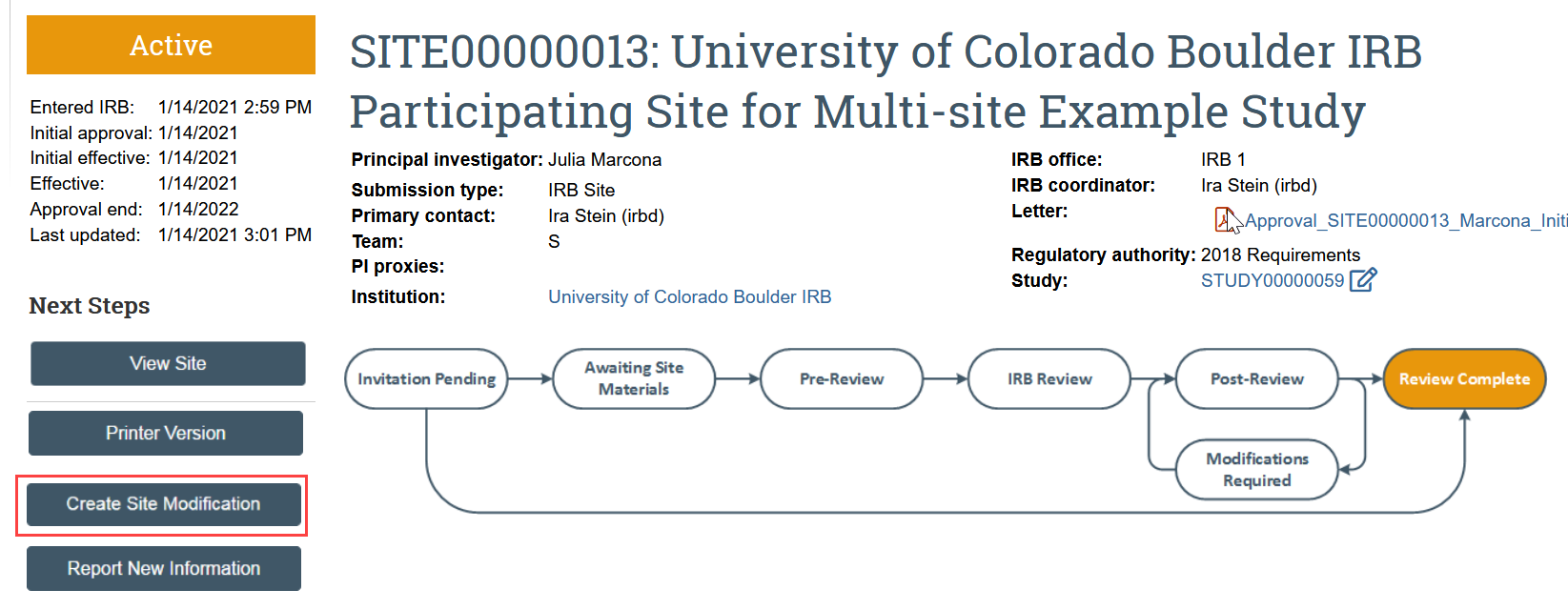
- Complete the Modification Information SmartForm and click Continue
- You are now in a draft version of the site SmartForm and should be on the Basic Site Information page. Update the draft version of the site to reflect any changes being made in the modification, by making all needed revisions to the Site SmartForms and uploading any revised or new documents.
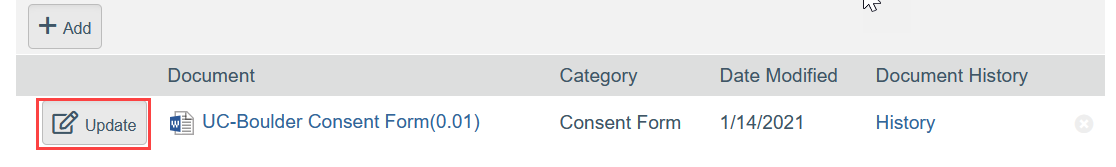
- To Update a Document:
- Click Update
- Select the revised file from your computer
- Click OK in the Edit Attachment window
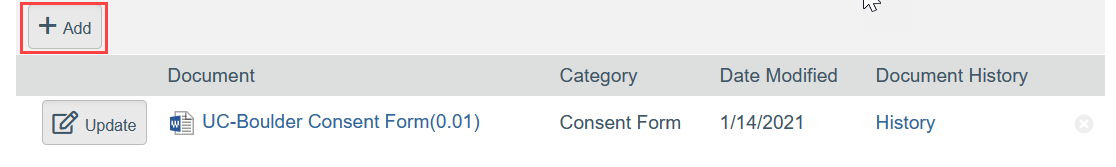
- To Add a New Document:
- Click Add
- Select the new document from your computer
- Click OK in the Edit Attachment window
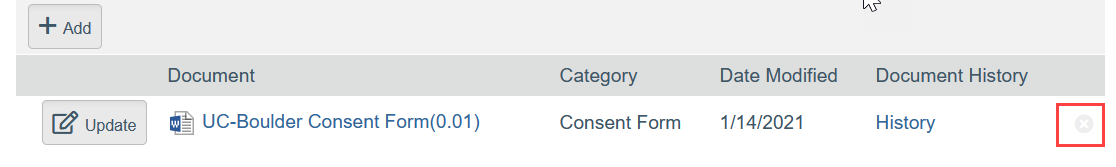
- To Remove a Document:
- Click the X by the document
- Click Save and Exit when you are finished editing the modification
- Click Submit in the modification workspace. The Submit activity must be completed once the modification is ready to go to HSD.
- Click OK to provide required verifications
The modification transitions to Pre-Review state and is now in HSD’s queue for review.
Communicating with HSD During Site Modification Review
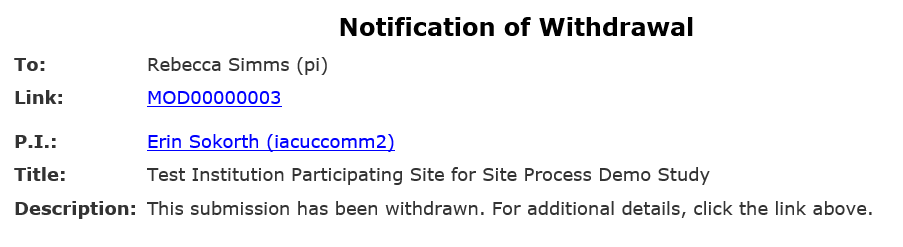
Site modifications do not have a formal clarification request process built in. Because of this, HSD will withdraw your site modification if more information is needed or a change is required before approving the modification. This pushes your site modification back to Pre-Submission. After completing any needed changes, you must resubmit to send the site modification back to HSD for review.
- If additional information or changes are required, HSD will withdraw the site modification and an automatic notification will be sent to the PI, any PI proxies, and the primary contact of the parent study
- Select Edit Modification and make any needed changes to the application
- Select Submit to send the site modification back to HSD for review
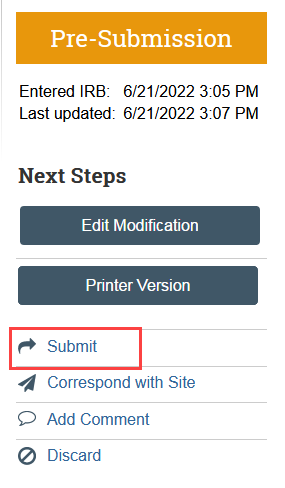
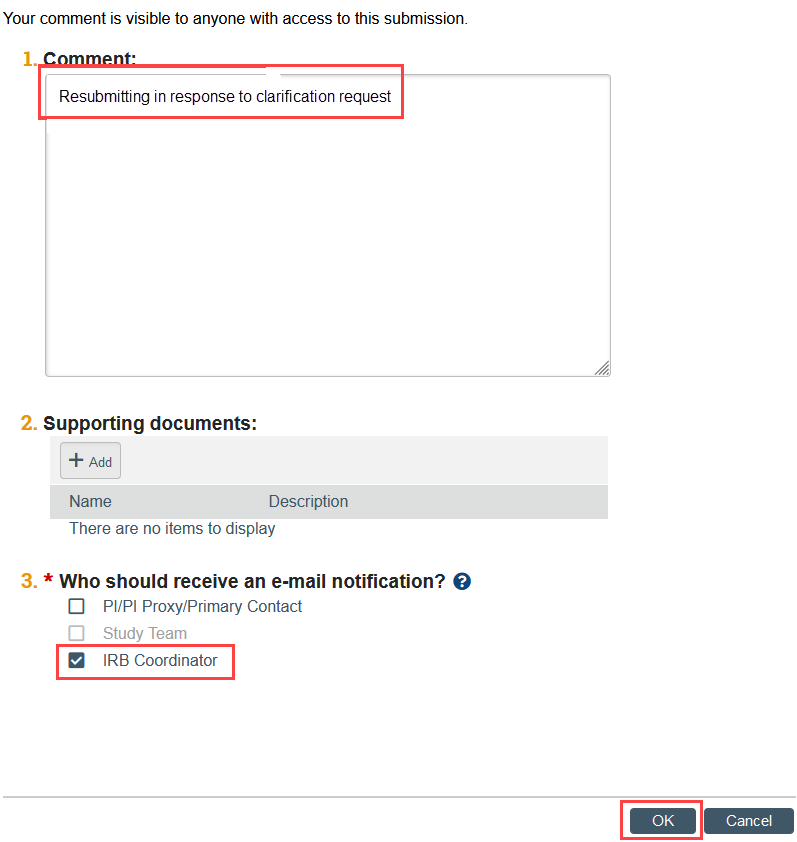
- Click Add Comment in the modification workspace. Indicate that you have provided the response to clarification and are resubmitting the Modification, check the box to notify the IRB coordinator, and click OK. This ensures that the correct IRB coordinator is notified.
After Modification Approval
After the modification is approved, the changes are published in the site record, so the site always contains the currently approved information. The UW PI, PI proxy, and primary contact also receive a notification containing the modification approval letter.