Human Subjects
Use this section to provide information about human subject involvement and approvals for the project to help reviewers determine compliance with UW and sponsor requirements. Contact the Human Subjects Division with questions.
Note: Zipline data will be refreshed each time you view this page.
As with other eGC1 pages, you can partially complete your entries and save the eGC1. You must complete the required fields before you route your eGC1 for approval.
Select Start Section to open the section initially, and then Edit Section to add or update existing data.
Human Subjects Questions:
HS-1. Will the project involve interaction with Human Subjects, or identifiable data or specimens from human subjects?
Answer: Yes or No. An answer is required.
HS-1A displays if HS-1 = Yes
HS-1A. Delayed Onset: Will IRB application submission be delayed until after award because you will (1) identify and develop new protocols that will be supported by the award or (2) complete other research described in the proposal that is necessary before the human subjects research can be fully planned.
Answer: Yes or No
HS-1B displays if HS-1 = Yes
HS-1B. IRB Approval Pending: Will you need to submit a new IRB application, modify an existing IRB application, or are awaiting approval of a current IRB application for the human subject research in this project?
Answer: Yes or No
A table for IRB Application Details displays if HS-1 = Yes
Note: If HS-1 = Yes, and HS-1A = No (Delayed Onset), and HS-1B = No (IRB Approval Pending), then you must include at least one IRB application. See details below on how to add an IRB Application.
HS-1C displays if HS-1 = Yes AND Sponsored Program Activity Type (Details page) is “Clinical Trial – Federal Sponsors” or “Clinical Trial – Non-Federal Sponsors”
HS-1C. CRBB Involvement. Will the project involve use of UW Medicine clinical services or clinical space as a site of patient care, or include medical treatment of a patient by a UW Physicians provider?
Answer: Yes or No
CRBB (Clinical Research Budget & Billing) Note: If any of the following statements apply to this proposed work, mark Yes, even if the research involves only usual patient care items or the study budget will not be charged.
-
- Some of the proposed work will be conducted in a UW Medicine clinical setting. See CRBB Coverage Analysis Checklist, Section II, Sites of Practice, for a complete list of sites. Studies using Seattle Cancer Care Alliance locations should use the SRAMP access point when submitting to CRBB.
- The study involves one or more clinical procedures at one of these sites, such as use of a patient examination room, blood draws, imaging, clinical lab tests, administration of a research medication, or procedures.
- Payment of physician professional fees for clinical services from a UW-affiliated or Northwest Hospital physician is required, separate from the salaried research personnel effort reflected in the budget.
Contact CRBB at crbb@uw.edu or 206-543-7774 for assistance.
HS-2. Stem Cells. Will this research involve the use and/or creation of human embryonic stem cells?
Answer: Yes or No An answer is required.
Add IRB Application
Start by selecting Add IRB Application. A dialog box will open where you can choose how you enter your data.
- Search Zipline
- Enter manually
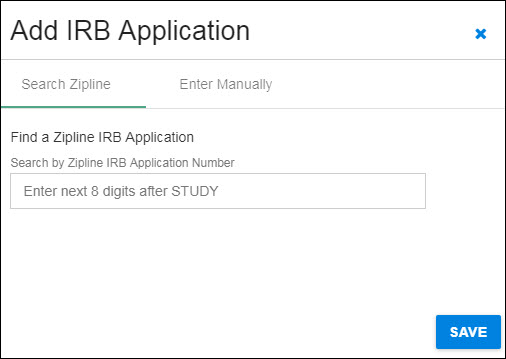
Search Zipline
You can use the blue “X” in the upper, right-hand corner to close the dialog and return to the Human Subjects section without saving any data.
The following image shows the initial dialog box.
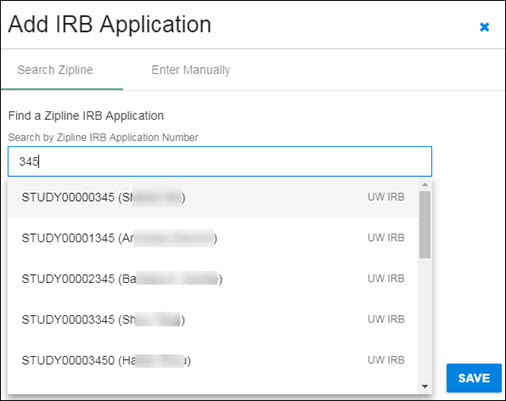
Enter all or part of the Zipline Application Number in the search box. The system will search for all applications that contain the entered numbers in the application number field. The results list will display below the search box, as shown in the following image.
Click on the appropriate study in the results list to select it. The study data will display in the dialog window, as shown in the following image.
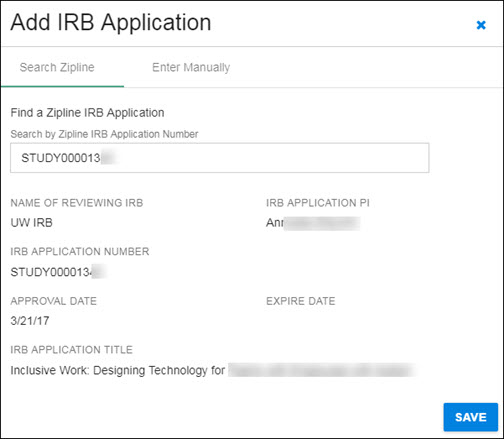
Use the Save button in the lower, right-hand corner to add the study data, and return to the Human Subjects section.
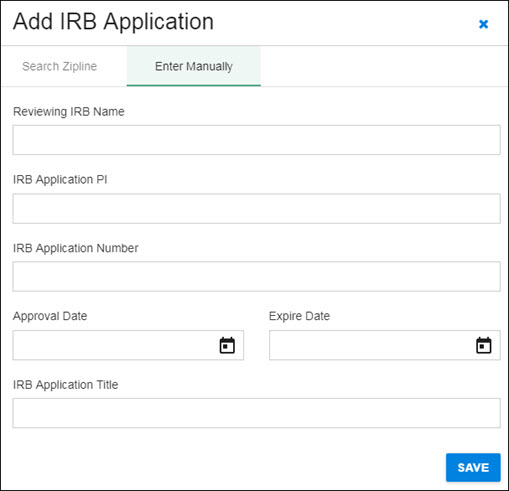
Enter Manually
You have the option to add IRB Application information manually. Select Enter Manually in the dialog, then add the requested information.
- Reviewing IRB Name
- IRB Application PI
- Reviewing IRB Application Number
- Approval Date
- Expire Date
- IRB Application Title
Select Save to return to the Human Subjects section.
Note: All of the fields are required prior to eGC1 completion.
The following image shows the manual entry screen.
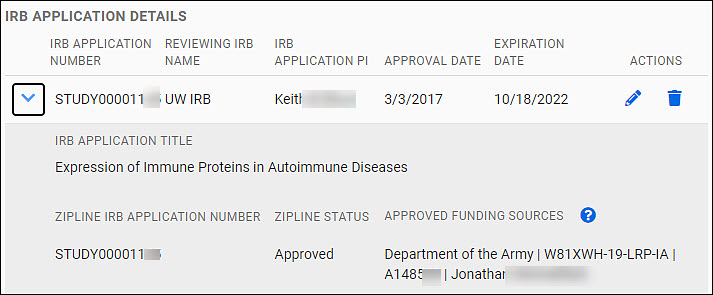
IRB Application Details section
Your data will appear as an expandable row of a table in the IRB Application Details section of the page.
The table row shows the follow data fields:
- IRB Application Number
- Reviewing IRB Name
- IRB Application PI
- Approval Date
- Expiration Date
You can edit or delete it using the icons (pencil, trash can) in the Actions column at the far right of the table row.
Use the chevron to the left of the row to expand the data shown. The additional data displayed includes:
- IRB Application Title
- Zipline Application Number
- Zipline Status
- Approved Funding Sources
The following image shows an expanded table entry.