If there is an existing application or award related to this eGC1, enter that information in this section.

| Field |
Description |
| Sponsor number at UW |
Enter the complete reference number (existing sponsor award number or previous eGC1 #) if this is a renewal, supplement, or extension application.
Include the year of funding this application represents (for example, you could indicate year 2 of a 5 year project as 2/5). Example: 5 R21 CA002176-02 2/5. |
| UW budget number |
Provide the UW budget number for the currently funded project. |
| Previous eGC1 number(s) |
Enter the previous eGC1 number(s), separated by commas. |
This section provides categorization information about the application. For guidance on which Project Type to select, review the How do I know what project type to select on an eGC1 and why is it important? FAQ. If you are unsure of how to answer these questions, please consult with the Principal Investigator or your local administrator.
You must select both a Project Type and a Sponsored Program Activity Type. The following image shows the Project Details section of the application.

| Project Type |
Description |
| Grant |
The application is for a specific investigator-initiated project with minimal programmatic involvement from the grantor (sponsor). See Glossary entry for details. |
| Contract |
The application’s funding mechanism is a contractual arrangement with specific deliverables and expected outcomes which benefit the sponsor. See Glossary entry for details.
Federal contracts: Route draft proposals for initial review to OSP no later than 7 business days before a sponsor deadline. See Why should I route an eGC1 for Contracts & Other Transactions to OSP ASAP? |
| Cooperative Agreement |
The application is for a federal assistance agreement mechanism that has substantial programmatic involvement by the federal agency. See Glossary entry for details. |
| Other Transaction Authority (OTA) |
The application is for a special award mechanism from certain federal agencies that is not a grant contract, or cooperative agreement. See Glossary entry for details.
Route draft OTA proposals for initial review to OSP no later than 7 business days before a sponsor deadline. See Why should I route an eGC1 for Contracts & Other Transactions to OSP ASAP? |
Note: Do not use an eGC1 for processing gifts. See Gift Processing for information on this topic.
Sponsored Program Activity Type
Select a value from the drop-down menu. It is organized by Institutional Activity Types: Organized Research, Instruction, and Other Sponsored Activity. For definitions, see GIM 13: Activity Types.
Note: if you choose the type “IPA/JPA/Staff Assignment” then the application is considered to be non-competing.
| Institutional Activity Types |
Sponsored Program Activity Types |
| Organized Research |
- Research: Basic
- Research: Applied
- Development
- Fellowship: Research Undergraduate
- Fellowship: Research Graduate/Professional
- Other Training: Research
- Clinical Trial: Federal Sponsors
|
| Instruction |
- Training and Development (UW Internal)
- Fellowship: Non-Research Undergraduate
- Fellowship: Non-Research Graduate/Professional
|
| Other Sponsored Activity |
- Professional Development/Public Service (UW External)
- Construction
- Equipment
- Clinical Trial: Non-Federal Sponsors
- Other Sponsored Activity
- IPA/JPA/Staff Assignment
|
Clinical Trial Phase
This field will display if you select either of the Clinical Trial values for SPA Type. Select the appropriate value. Choices are: I, II, III, IV, and Other. For definition of these choices, see the Clinical Trials web page.
SAGE allows you to create two types of eGC1s – standard and Grant Runner. The Grant Runner version contains all of the same pages as a standard eGC1 and also includes the integrated form package for the electronic submission directly from SAGE via Grants.gov to NIH. Currently, you may only use Grant Runner for opportunities sponsored by NIH and its individual institutes. See the full list of supported NIH Activity Codes.
Using Grant Runner is optional. You can also submit using ASSIST.
To create either type of eGC1, start by clicking on the Create New eGC1 button on the My eGC1s page to start the Wizard.
For the question “Do you want to see if your application is eligible?” click Yes and then click Next to use with Grant Runner. You will then continue with steps 2-5 of the Grant Runner Wizard (described below). The data collected in these steps will pre-populate some fields on both eGC1 pages and sponsor forms.
If you want to create a standard eGC1, click No and then click Next. On the confirmation page, you can return to the Grant Runner Wizard or click Create Standard eGC1 to create your eGC1.
Note: For guidance on completing an NAA type application, see OSP’s NAA eGC1 Instructions FAQ.
Additional Grant Runner Wizard Steps
Steps 2-5 of the Wizard gather specific information to:
- Confirm eligibility to use Grant Runner.
- Pre-populate data in the eGC1 and the Grant Runner sponsor forms.
Step 2: Sponsor
Use the Look Up Sponsor button to search for and select the sponsor. Currently, you may only use Grant Runner for opportunities sponsored by NIH and its individual institutes.
If you have selected any of the NIH institutes, when you click Next, the Wizard will continue with Step 3; for all other sponsors, the Wizard will present options to either create a non-Grant Runner eGC1 or return to the My eGC1s list. For more information, see Sponsor.
The sponsor displays on the eGC1 Details page. Once you complete the Wizard, you cannot change the sponsor.
Step 3: Opportunity ID
Use the Look Up Opportunity button to search Grants.gov for the Opportunity ID as listed on the sponsor instructions.
If SAGE supports the form set, the opportunity will appear in the results list. Click “Select” to the left of the opportunity to choose it and add the sponsor forms to the eGC1.
If SAGE does not support the form set, that will be indicated to the left of the opportunity in the results list. You can then “Cancel” out of the look up, and either cancel the creation of the eGC1 by clicking “Back to My eGC1s” or click on the “Cancel Wizard and create a standard eGC1” link.
If the opportunity cannot be found, the message “Sorry, no results for your search.” will display. In that case, check that you have properly entered the opportunity ID.
The Wizard presents options to either create a non-Grant Runner eGC1 or display the My eGC1s list.
Note: The Opportunity ID is also known as the Program Announcement Number (PA) or Request for Proposal (RFP).
The opportunity information, including opening and closing dates, is displayed on the eGC1 Abstract & RFA/RFP page once the application is created. Once you complete the Wizard, you cannot change the opportunity.
Step 4: Principal Investigator
Use the Look Up Principal Investigator button to search for and select the Principal Investigator. The name and directory data will display. Several fields are editable:
- Title
- UW box number
- Phone
- Fax
- Cell Phone
- Pager
- Email
The values will be displayed on the eGC1 PI & Personnel page and some of the Grant Runner forms. Prefix and Suffix values can be added, and editing can be done, on the eGC1 PI & Personnel page after the Wizard is complete. For more information, see Principal Investigator.
Step 5: Application Details
Enter values for each of these required fields:
- Full Application Title
- Requested Start Date
- Requested End Date
- Application type
This data will display on the eGC1 Details page and some of the Grant Runner forms. You can edit these on the eGC1 Details pages after the Wizard is complete. For more information, see Application Details.
When the wizard is complete, the standard pages for the eGC1 will display, along with the mandatory and optional Grant Runner forms.
You can access this read-only summary of the eGC1 from within the eGC1 or from the Approval Flow pages. It is designed to assist approvers by presenting essential approval-related information on a single page.
For a comprehensive view of all data on the entire eGC1, go to the Approval Flow page and select View eGC1. A preview screen will appear which you can view online or printe.
The Application Details section includes:
- eGC1 Number: the system-generated unique number for the application
- Long Title (Full Application Title)
- Short Title: identifies the project in the financial system
- Last completed timestamp
- Requested Start Date
- Requested End Date
- Sponsor deadline: The last date the sponsor will accept the application
- Application Type: New, Competing Renewal, etc.
- Sponsor Name
- Originating (Prime) Sponsor, if applicable
- Org Code Receiving Funding: The ten-digit code for the unit managing the funding
The Principal Investigator section displays the name of the PI.
The Activity Locations section displays the list of locations where the research activities will occur. The values listed are: Name, Source, Location Type, and Rent Charged to Sponsor.
The Space Planning section displays the response to the question on the Activity Locations page.
The International Involvement section displays the response to the question on the Activity Locations page.
The Budget & Fiscal Compliance section includes:
- The responses to the three Fiscal Compliance questions
- F&A (Facilities & Administration) Cost Rate
- A table of costs for the current budget period and the total for all periods for:
- Total direct costs
- Total F&A costs
- Total costs
The Cost Sharing section displays the total cost sharing for all periods. This includes all amounts from UW sources (personnel and non-personnel), third party sources, and unrecovered indirect costs. It also shows the UW Summary by Unit information from the Cost Sharing page.
The Compliance Questions section lists each of the Non-Fiscal Compliance areas with a value of Yes or No. The value is Yes if any questions within that section are answered “yes”.
The eGC1 Campus Comments section displays what was entered in the Additional Information section of the Certify & Route page of the eGC1.
New Features
SAGE eGC1s and Approvals Search Enhancements
Users will see a few new search features available to them, on the eGC1 and Approvals task lists.
- Users will now be able to jump more directly to a particular eGC1 from their My eGC1s or My Approvals pages, using a new eGC1 search field located on the task lists.
- Users can now search in My Approvals by personnel name. Search results will include all eGC1s for which they are an approver or watcher, where the chosen person is listed on the PI & Personnel page regardless of role type.
- The PI Name field has been added back to the My eGC1s Advanced Search tool, to allow users to quickly enter all or part of a PI’s name and initiate a search. This feature was removed in January, when the Personnel Search feature was added, but by user request, both fields will now be available for those that like the speed of being able to quickly find eGC1s by PI name.
- When a user performs an Advanced Search from the My eGC1s task list, they will be able to export their search results to Excel.
Addition of University District Building to Location List
- The University District Building has been added to the off campus locations list found on the FG-2 compliance explanations section of the eGC1. It will now be easier for eGC1 Preparers to select that building when projects are eligible for the location designation of “off campus”, and will no longer need to enter the location address.
Suppression of HSD JIT and GIM 19 notifications for ATF and Industry Sponsored Clinical Trail eGC1s
- Human Subjects Just-In-Time email notifications and GIM 19 deadline email notifications will no longer be sent to contacts for eGC1s that are after-the-fact or industry sponsored clinical trials.
Updated handling when users attempt to remove themselves from an eGC1 or Budget
- When users remove themselves from an eGC1 or budget, they will now receive a warning, alerting them that their access to that eGC1 or budget may be affected. When users remove their last access to an eGC1 or budget, they will now be redirected back to their task list.
This pop-up window displays the investigator’s disclosure history for the past year. It displays when you click the Investigator Disclosure History link for a specific investigator on the PI & Personnel page.
The Investigator section lists the person’s name and employee number.
The One Year Disclosure History section displays a table of disclosures with the following information.
| Field |
Description |
| Disclosure Submitted |
The date the investigator completed this disclosure. |
| Disclosure Number |
The unique number assigned by the Financial Interests Disclosure System (FIDS) to the disclosure when it was created. |
| Primary eGC1 |
The number of the eGC1 associated with this disclosure. If the disclosure is for C4C, IRB, or SFI-only then “(not available)” is displayed. |
| Review Status |
The current status of the disclosure. |
| FCOI Status |
The result of the disclosure’s review. |
If an investigator has never completed a disclosure in FIDS, or has not completed one in the last 12 months, the following message will display:
- This investigator has not submitted any disclosures within the past year.
Fixes/Enhancements
In SAGE Budget, we revised how calculations are made for salary distributions.
- In the March 2013 release, we revised salary calculations to include additional earn types. We found a few rare cases where SAGE Budget lists inaccurate salaries when the salary distributions have not yet been entered into the HR database. We have now revised SAGE Budget to account for the distributions which are not in the database.
We updated our tools’ styles.
To ensure that our tools have a more consistent look and are easier to maintain, we made some changes in our code. In some newer browsers, you may notice slightly different colors, rounded corners, and other minor changes. The changes were made in the following tools:
- SAGE – System to Administer Grants Electronically
- FIDS – Financial Interest Disclosure System
- SPAERC – Sponsored Projects Administration & Electronic Research Compliance (for OSP)
- SERA – System for Electronic Research Accounting (for GCA)
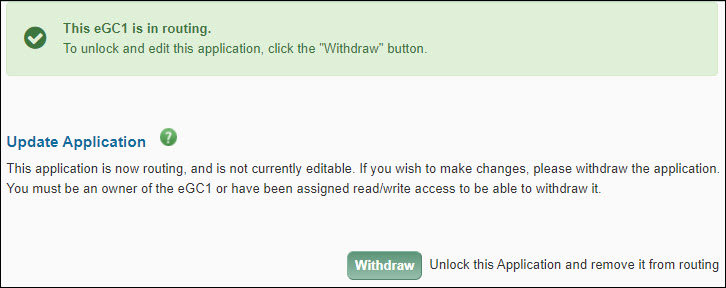
This section appears at the top of the Certify & Route page after an application has been routed to reviewers.
To unlock your application for editing, click the Withdraw button to change its status to Withdrawn and make it editable. Only eGC1 owners, users with assigned read/write access, or reviewers with the Global Editor role can withdraw an eGC1.
After you make your changes and re-route the eGC1, it will continue routing where it stopped when you withdrew the application. Thus, people who already approved the eGC1 do not need to approve it again.
Depending on the significance of the changes made, you may want to use the Add Approver feature to add them to the flow a second time, so they can approve the changes. Check with your unit for guidance.
If you make changes to a compliance question, change the PI, or add new personnel, etc., the approval graph may change. Any approved nodes that remain on the changed flow, will still be in an approved state.
Note: If you have marked an eGC1 as Ready to Submit and it has In OSP status, you cannot withdraw it. Instead, contact OSP and request they return the eGC1.