Upgrade Changes FAQs
Topics
- New Feature: Dashboard
- New Feature: Site Search
- New Requirement for Student and Resident Principal Investigators: IRB 101 Tutorial
- Other Changes
New Feature: Dashboard
What is the Dashboard?
The Dashboard link has replaced the My Inbox link in Zipline. All users now default to the Dashboard after logging into Zipline. The Dashboard contains:
- The 10 most recently viewed submissions;
- Any submissions that you have pinned for quick access;
- My Inbox, which shows submissions that require action from you or your team;
- My Reviews, which is most useful for faculty advisors and IRB committee members;
- The create menu, used to create new studies and reports of new information; and
- Quick links, which have been shortened to IRB Home and IRB Reports. Select IRB Home to go to the main IRB menu, which contains the Submissions area and the Meetings area.

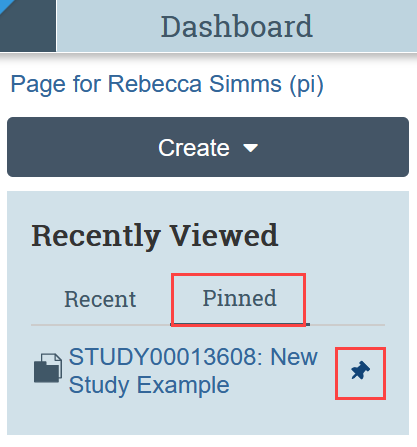
What are pinned studies, and how do I pin a study on the Dashboard?
With the upgrade, users may now save, or “pin,” submissions for quick access. Submissions can only be pinned if they are in the “recently viewed” column. To pin a submission, simply select the pin icon next to it. To unpin a submission, deselect the pin icon.
What shows up in the My Reviews tab?
The My Reviews tab is most useful for faculty advisors and IRB committee members. It only shows studies that have been assigned to you as a faculty advisor, or, if you are an IRB committee member, studies that have been assigned to you as a primary or secondary reviewer. Note that any studies assigned for faculty advisor or IRB committee review will also show up in My Inbox. For most users, nothing will appear in the My Reviews tab.

How do I create a new study now?
The buttons to create a new study or report of new information have been moved into the same menu. Select the “Create” button and choose the type of submission you’d like to create.

How do I find studies that aren’t on My Dashboard?
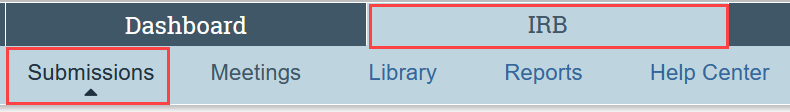
The submissions area contains all submissions that you have permission to view. To go to the submissions area, select the IRB tab in the main menu, and then select Submissions.
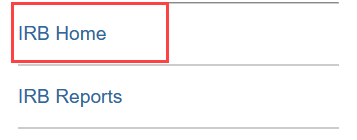
Alternatively, you can use the quick links on the side to select IRB Home and then select Submissions.
New Feature: Site Search
Where do I find the new site search feature?
The new site search feature is available in the Submissions area in the top right portion of the screen. Select IRB in the top menu, and Submissions in the submenu. You will not find it on your Dashboard.

What are some tips for using the new site search feature?
The site search feature, available in the Submissions area, allows you to search all of your submissions in Zipline, including documents uploaded to the SmartForms. Site search is most useful when using specific search terms, such as a grant number, DORA application number, or study drug name. We recommend searching Projects to avoid old document templates that may appear. Click the help icon next to the search bar for extensive search tips.
New Requirement for Student and Resident Principal Investigators: IRB 101 Tutorial
What is the new requirement for student and resident principal investigators?
Students and residents who are listed as the principal investigator (PI) on an IRB application are required to take a one-time, on-demand, e-learning tutorial about getting started with the IRB. The IRB 101 tutorial covers the basics of human subjects research and provides important practical information about navigating the IRB process at UW. The tutorial takes approximately 60 minutes to complete. HSD staff may begin application review before the training is completed, but the student or resident PI must provide verification that the training has been completed before an IRB application can be approved or given an exempt determination. See the FAQ on the IRB 101 tutorial page for more information, including how to provide your training documentation to the IRB.
Are fellows and post-docs required to provide IRB 101 training documentation or get faculty advisor review?
No. Fellows and post-docs are not required to provide IRB 101 training documentation or get faculty advisor reviews on their IRB applications. Fellows and post-docs are still encouraged to view the IRB 101 training, as it is designed to be helpful for those new to working with the UW IRB.
Other Changes
What are other system changes that may be of interest?
The primary change that may be of interest is the return of breadcrumb navigation within a submission record to easily return to the parent study from a follow-on submission record.

Additionally, some information in the study record summary has been rearranged slightly. However, no fields have been removed or added.
Also, there are some minor changes to the electronic application (SmartForm) questions, including the removal of the conversion application question and a revision to the student principal investigator question to align with the new IRB 101 training requirement.
Where can I find a complete list of upgrade changes?
See the Release Notes for a full list of changes.
