Application Process FAQs
Topics
- General
- Ancillary Review
- Funding
- Study Team
- Do I need to add my entire study team on the Local Study Team Members page in the SmartForm application?
- The study coordinator changed. What do I need to do?
- The PI is changing. What needs to happen with the Zipline application?
- I need to make a change to the study roles for an older Zipline application that has the ADDENDUM: Study Roles form. How do I make changes to study roles for older applications?
- Responding to HSD
- Notifications
- Modifications
- RNI
General
Why hasn’t IRB review started yet?
If the item is still in Pre-Submission, you have not yet submitted the item to HSD. After you have completed the application forms and SmartForms, the PI or PI proxy must click the Submit button for IRB review to begin.

If the item is in Pre-Review, it is in HSD’s queue for review. Contact the team that reviews for your department for more information.
I can’t find my PI/faculty advisor/study team member in the system. How do I find them?
The person who creates the study in Zipline is automatically listed as the PI and can be changed by clicking the “ellpsis” button next to the PI question on the Basic Information SmartForm (the first web form of the application).
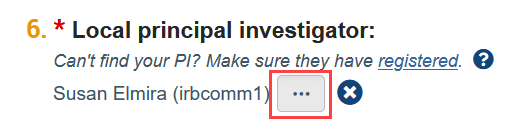
If the PI is not listed in the selection window that appears, it means they have not yet registered in Zipline. Similarly, if the faculty advisor is not listed in the selection window that appears when adding an ancillary review or a study team member is not listed in the selection window on the Local Study Team Members page, it means that they have not yet registered in Zipline. Please see Account Creation and Management for more details. Once a person has registered, they will show up as a selection option in Zipline.
I’m not sure how to answer a question on the SmartForm or the IRB Protocol Form. What should I do?
For SmartForm questions, check the related help bubble for more guidance about how to answer the question.
In the IRB Protocol, start by taking a look at any instructions or examples embedded within the form to help answer your questions.
If you are still unsure how to proceed, contact the HSD Team that reviews for your department or hsdinfo@uw.edu for additional guidance.
What’s the difference between withdrawing and discarding an application?
Withdrawing an application pulls it back to Pre-Submission so that you can make edits and then resubmit it to HSD for review. Discarding an application pulls it from review permanently so that it can no longer be edited or reviewed. For more information see Withdraw or Discard.
Ancillary Review
My faculty advisor can’t submit ancillary review. What should I do?
First, make sure that the faculty advisor was added as an ancillary reviewer. In the study workspace, click Manage Ancillary Reviews under Next Steps. If you do not see your faculty advisor as a reviewer, you must add them. See Add Faculty Advisor Review for more information.
If your faculty advisor was added as an ancillary reviewer but does not see Submit Ancillary Review as an option for your study, make sure that your faculty advisor is not listed on the Local Study Team Members page in the study SmartForm. If your faculty advisor is listed on this page, removing them will correct the issue. If your study application is not in an editable state, contact the assigned IRB coordinator or the HSD team that reviews for your department for assistance.
Funding
What do I do if my sponsor is not listed as a funding organization in Zipline?
You can search for part of the name of your funding source using the % symbol (e.g., if you’re looking for the ‘Patient Centered Outcomes Research Institute (PCORI)’ you could search ‘%Patient’ ‘%Patient-Centered’ ‘%Research Institute’, etc.).
If your funding is internal to the University of Washington (e.g. internal department funds), the funding source will not be listed in Zipline. Select instead “OSP Not Involved”. Write N/A for the Sponsor’s Funding ID and Grants Office ID.
If your funding is a gift disbursed through the University of Washington, the funding source will not be in Zipline. Select “Gift through the UW” instead. Write N/A for the Sponsor’s Funding ID and Grants Office ID.
If your funding source is not internal to the UW, is not a gift, and does not appear on the list, contact HSD staff at hsdinfo@uw.edu before you complete your application, so it can be added to the list for you to select.
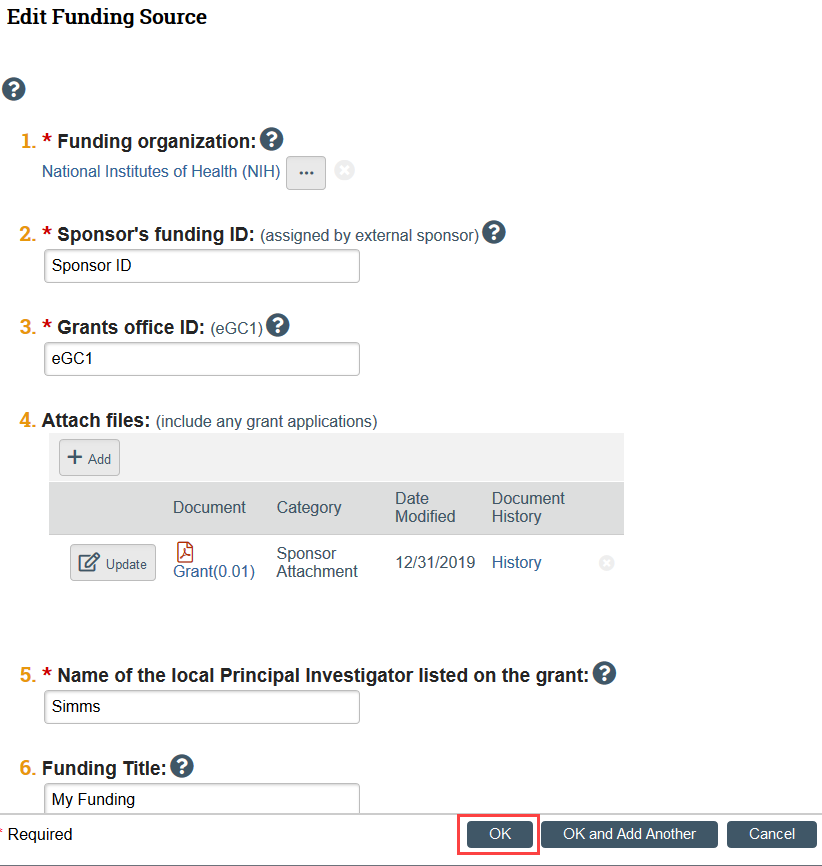
I added a funder in Zipline, but now I need to make revisions. How do I edit the funding source?
Once you have added a funder, click on the Funding Source name to open the specific funder information.

A slide-in will appear and you can edit the funder information from there.
Click OK when you have finished editing.
Study Team
Do I need to add my entire study team on the Local Study Team Members page in the SmartForm application?
No. Only those who need viewing and editing privileges and those who will be a PI proxy must be listed on this page.
The study coordinator changed. What do I need to do?
To change Zipline access only: Submit a study team member modification to update who should be able to access the application.
If study team qualifications or other documents, such as consent forms, are also changing: Submit a modification to change both the study team member page and other parts of the study. See Study Modifications for more information.
The PI is changing. What needs to happen with the Zipline application?
The new PI will not have access to the Zipline application until a study modification updating the PI is approved. If possible, we strongly recommend having the former PI designate a PI proxy to ensure that someone on the study team is able to submit a modification and respond to any questions from HSD. If the former PI is no longer available, contact the HSD team that reviews for your department for assistance.
I need to make a change to the study roles for an older Zipline application that has the ADDENDUM: Study Roles form. How do I make changes to study roles for older applications?
The ADDENDUM: Study Roles form was retired with the January 2020 Zipline upgrade and should no longer be updated during modifications to existing Zipline applications. If you need to make changes to study roles for an older application that includes the retired addendum on the Contacts tab, let the team that reviews for your department know.
HSD staff will provide you with a very short form that you should fill out to reflect the current state of the study team. This means that you may need to include information that already exists in the ADDENDUM: Study Roles, which is still accessible in the study. The reason we ask for this is so that moving forward, we have all the information about study team qualifications in one place. This document should be uploaded to the IRB Protocol question on the Basic Information page of the Zipline application and updated for all future revisions to the study team roles or qualifications.
Responding to HSD
I can’t find the Submit/Submit Response button. How do I send the application to HSD for review?
Look for the ‘Submit’ or ‘Submit Response’ button on the left hand side of the study workspace under Next Steps. Only the PI or PI Proxy has the ability to submit the study or to submit a response to HSD requests for clarification. For instructions on how to add a PI Proxy to your study, see Assign PI Proxy.
I submitted my application, but I forgot something. How do I make a change right now?
If the application was just submitted, you can withdraw your study during Pre-Review to change something you have forgotten. However, you will have to re-submit your study after you have made the changes, and if HSD staff started review, it will have to start over when the application is resubmitted.
If review has started, contact the assigned IRB coordinator. The IRB coordinator can request changes to push the application to you for further editing.

Should I use a comment to contact HSD about my application?
In general, HSD does not recommend using the Add Comment button if you need to contact HSD staff about your application.
![]()
HSD staff do not routinely monitor applications for comments. If you have a question about your application, the best way to ensure a prompt response is to email the team that is assigned to your study. If no team is assigned, email hsdinfo@uw.edu.

The Add Comment button cannot be used to respond to a clarification request from HSD because it does not push the review forward in the system. If the application is in Pre-Submission or Clarification Requested state and you need to push it to HSD so that the review can move forward, use the Submit or the Submit Response button. This must be completed by the PI or a PI proxy.
Notifications
What automated notifications does the PI receive from Zipline?
The PI, PI Proxy, and Primary Contact will receive notifications when:
- HSD staff request clarification during review of an application or Report of New Information
- HSD staff send a formal letter with the study approval, conditional approval, deferral, or determination
- HSD staff confirm reliance or record the reviewing IRB’s decision for an external IRB application
- HSD staff close, suspend, or terminate the study
- HSD staff administratively withdraw the study or Report of New Information
- The deadline for submission of a status report is approaching (notification sent at 60 and 30 days prior)
- Studies that are in “Pending sIRB Review” state receive a reminder to submit the approval letter every 6 months
- External IRB studies receive an annual reminder to update or close the study as needed
- UW Reviewed studies that do not expire receive an annual reminder to submit study modifications and RNIs
- The study approval lapses
- The study team has not responded to requested clarifications within 30 days
- A study team member is assigned as a Responsible Party for a Report of New Information (requires action by the study team member)
- A Report of New Information is acknowledged by HSD
- A comment is added to the study, and the PI, PI Proxy, or Primary Contact is/are selected as a recipient
Modifications
Why are there 2 types of modifications?
Zipline asks you to indicate the modification scope when you are submitting a new modification. The options are “Study team member information” and “Other parts of the study.” You can select one or both options. Zipline will not let you submit a second modification with the same scope until the first one is discarded or approved.
Study team member modifications are intended to be quick administrative study changes that simply update who is able to view and edit the study record in Zipline.
For any other study change, including changes to the study roles, you must indicate “other parts of the study” for the modification scope. This triggers the standard IRB review process.
What should I do if I selected the wrong modification scope?
The page that indicates the modification scope is not editable once you’ve progressed past that page.
Option 1: Discard the modification that has been created and start again. This option is only recommended if you have not begun making your changes in the modification or if you selected the incorrect modification scope initially.
Option 2: Create a second modification and check the needed modification scope box there. Zipline allows a second modification to be created if the scope is different from the modification currently in review.
RNI
I need to edit a RNI that someone else on the study team submitted. How do I do this?
In general, the person who submitted the RNI is the only one who can make edits or submit responses to HSD- PI proxies are not available for RNI. If the person who submitted is no longer available, contact hsdreprt@uw.edu for assistance.
What’s the difference between a RNI and a study modification?
A report of new information (RNI) is used to report new information or events to HSD’s compliance office for review. For example, breaches of confidentiality, unanticipated problems, and DSMB reports should all be submitted as RNI. See SOP RNI Reporting by Researchers for more information. Study records cannot be updated with a RNI, so if a change to the study is needed, a study modification must also be submitted.
