Overview of IRB Processes
Table of Contents
- UW IRB Reviewed Study, Continuing Review, and Modification Review Process
- Site Review Process
- Reportable New Information Process
- Ancillary Review Process
- External (Non-UW) Request Process
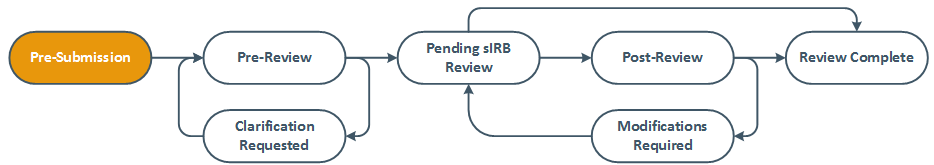
At the highest level, all Zipline submissions follow the same basic workflow. The actions available for each study are dependent on the submission state as well as the role of the user. For example, study staff cannot make additional edits when a study is in Pre-Review.
The system shows the diagram below when you view an individual submission and shows the current state in orange. See Submission States to learn more about what each state means.

UW IRB Reviewed Study, Continuing Review, and Modification Review Process
The basic process for all UW reviewed studies is shown in the following diagram. Modifications and continuing review reports also follow the same basic process. Multi-site and collaborative studies also require a separate review for each non-UW site if the UW IRB is reviewing the activities of other organizations or institutions. The exploded view shows what occurs during the IRB review process.
The legend indicates who can take major actions during each state within the process. Keep in mind that the diagram does not show all possible paths that a submission may take. The diagram shows the most likely path through the review process with common options identified.
Site Review Process
Site review is required for multi-site and collaborative studies where the UW IRB is reviewing the activities of other institutions. The overall protocol and procedures are reviewed during the study review process, while any site-specific considerations (such as applicable state laws) and materials (such as site-specific consent forms) are reviewed during the site review.
After the parent study is approved, HSD Staff create the Zipline site submissions. The study team edits the site submission to add the site application and any site-specific materials. There is no formal clarification request process for site submissions. Instead, once the site submission is ready for HSD, the study team notifies HSD by adding a comment to it. If any additional information is needed, HSD notifies the study team by adding a comment to the site submission.

Once sites are approved, there is no formal clarification request process for site modifications. Instead, if additional information is needed, HSD will withdraw the modification so that the study team can edit and resubmit.

Sites do not go through their own continuing review- instead they are included in continuing review for the overall study.
Reportable New Information Process
Reports of New Information (RNI) may be submitted by anyone with access to Zipline. RNI may be associated with no studies, one study, or multiple studies. They may also be associated with any modifications or sites for an associated study.
Unlike other submissions, the person who submits the RNI is the only one with access to make changes and respond to HSD.
The RNI is routed differently depending on the significance of the determination that HSD staff make during review. Depending on the determination, it may not require any additional review, a designated reviewer acting on behalf of the IRB may review it, or it may require review from the convened IRB.

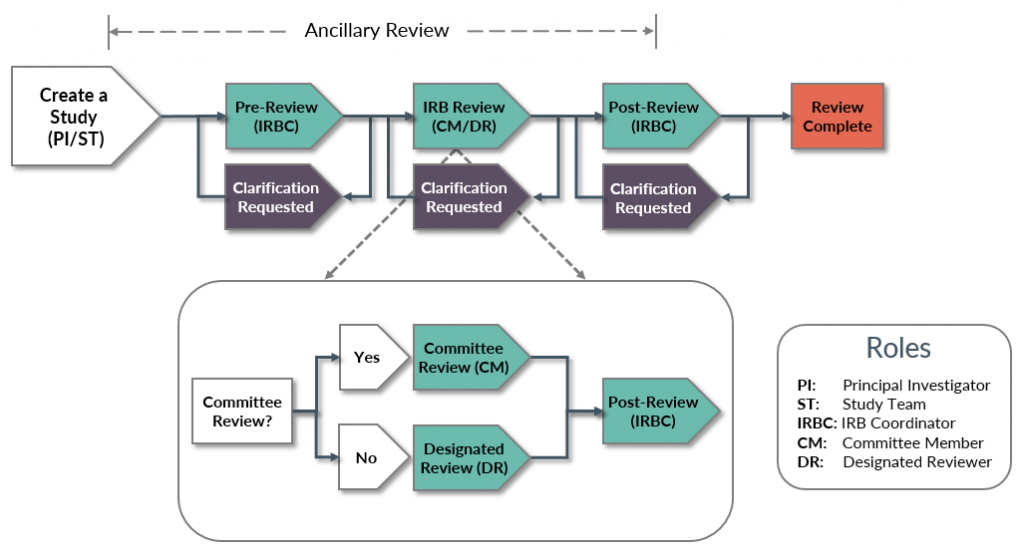
Ancillary Review Process
Ancillary reviews allow individuals, departments, and other organizations to give feedback on the study in parallel with the IRB review. For example, faculty advisor review of a new study is considered an ancillary review in the Zipline system.
Ancillary reviews can occur at any time from the Pre-Submission through IRB Review states, as illustrated here. Once it is complete, ancillary review feedback is visible on the Reviews tab to everyone who can access the submission.

External (Non-UW) Request Process
All UW studies that will be reviewed by a non-UW IRB must go through the external IRB request process in Zipline. Instead of providing IRB review for the study, HSD staff determine whether or not external IRB review is appropriate for the study and work with the reviewing IRB to set up a reliance agreement.
Once HSD staff confirm the reliance, the UW study team receives an acknowledgement letter and the study moves to Pending sIRB state in Zipline. The study will remain in Pending sIRB state until documentation that the reviewing IRB has approved the study is provided to HSD. Once HSD staff receive documentation of study approval, the Zipline record will be updated, and the study will be considered Active.