A Report of New Information (RNI) is used to provide the IRB with any issues or relevant new information. See SOP RNI Reporting by Researchers [2] for more information about what should be reported.
If you need to submit a PAVE report, see PAVE Reports in Zipline [3] for more specific instructions.
The RNI submitter is the only person who can respond to any clarification requests about the RNI. The RNI may be linked to one or more studies, sites, or modifications when the RNI is submitted, but it is not required. HSD can also link studies, sites, and modifications to a RNI at any time during review. The RNI submitter has full access to edit the RNI. However, the PI, any PI proxies, the primary contact, and study team members of all related (linked) studies are given read-only access to the RNI submission. The PIs, PI proxies, and primary contacts receive the same e-mail notifications as the RNI submitter throughout the workflow.
Table of Contents
- Submit RNI [4]
- Respond to Action Required [5]
Submit RNI
1. Click Report New Information from My Inbox, or from a related study or site
2. Enter a short title for the submission
- NOTE: For most submissions we recommend using the following format: [PI Name]_[1-2 Word Descriptor of Event], e.g. Smith_Tachycardia SAE, Smith_DSMB Report, Smith_Revised IB
3. Enter the date you became aware of the information
4. Select the category (or categories) that best represents the new information being reported

5. Describe the event and what, if any corrective action has been taken or is proposed
- NOTE: If a related modification will be submitted, please note this here. If the modification has already been created, make sure to associate (link) it with the RNI.
- NOTE: If the RNI involves safety monitor or DSMB reports OR research-related problems or events, write, “See attached RNI Supplement” and upload the completed RNI Supplement form as a supporting document.
6. Indicate your assessment of the RNI

7. Associate (link) any modifications [6] (if created) you are proposing because of the RNI and associate (link) any study(ies) that the RNI relates to
- NOTE: To add a related modification, first add the modification’s parent study. After adding the parent study, the RNI SmartForm must also be saved before the modification can be added.
- Click the ellipsis

- Select the checkbox for the related study or modification and click OK

8. Upload the RNI Supplement [7] as a supporting document if the RNI involves safety monitor or DSMB reports OR research-related problems or events
9. Click Save and Exit to leave the SmartForm
10. Click Submit RNI and click OK to complete required verifications
- NOTE: The RNI can only be submitted by the person who creates it.

The RNI transitions to Pre-Review state and is now in HSD’s queue for review.

Once HSD and the IRB have reviewed the RNI, the submitter will receive a notification that:
- HSD and the IRB have made a final determination and no additional action is needed at this time; OR
- HSD and the IRB requires more information or additional action
Respond to Action Required
After reviewing a Report of New Information (RNI), the IRB may require specific actions to be taken in response to the reported issue. A responsible party is assigned by the IRB to complete the action. The responsible party can respond using the Submit Action Response activity when the action has been completed.
The system sends an email to notify the responsible party and the submitter of the RNI, as well as the PIs, PI proxies, and primary contacts of all related studies. The RNI also appears in My Inbox for the responsible party.
1. View the details of the RNI submission and the IRB’s required action plan, as described here:
- Read the letter: Click the letter link near the top of the RNI workspace. The letter typically contains the IRB’s required action plan and a summary of the IRB’s decisions.
- Review the action plan: Click the Action Plan tab and read the required action plan listed there, plus any history of the action plan that might be helpful.
- Review the RNI submission details: If you aren’t already familiar with the details of the RNI, read it by clicking View RNI on the left side.

2. Take the actions required to complete the action plan
- TIP: If the action plan requires a change to a study, create a modification and submit it for review [6]. If there is a related modification, you should also link it to the RNI using the Add Related Submission activity.
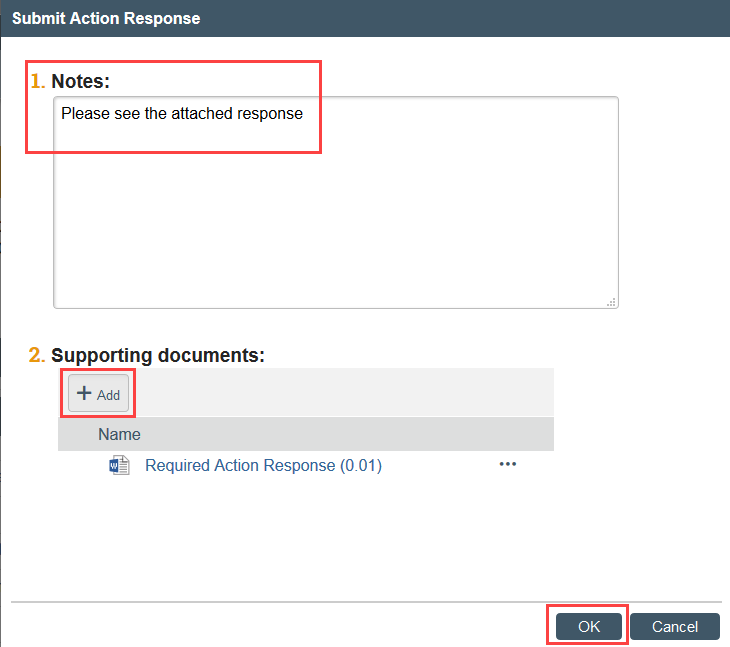
3. Prepare your written response as a document to upload or copy and paste into the activity
4. Click Submit Action Response to indicate that the action plan is complete

5. Add your written response in the notes field or as an attachment, including a summary of the actions taken to resolve the reported issue and complete the action plan and click OK