Continuing review is conducted at the study level. Any information about other institutions relying on the UW for review [2] should be reported with the overall study continuing review.
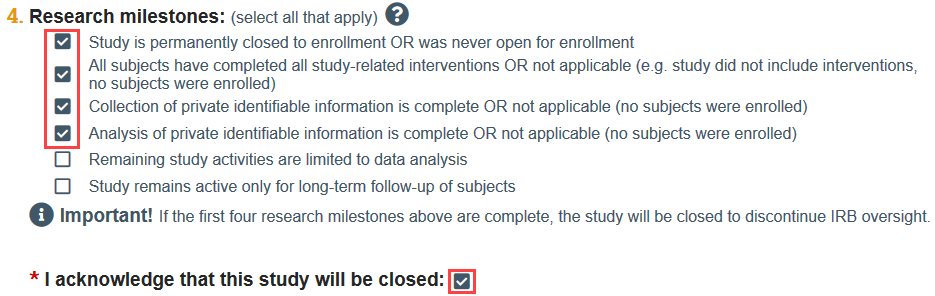
The continuing review report is used both for study renewal and study closure. When you indicate on the continuing review SmartForm that the first four research milestones have been met, Zipline prompts you to acknowledge that the study will be closed. These milestones indicate that all enrollment, interventions, and analysis of subjects’ private identifiable information are complete.
The continuing review report has two parts:
- The Continuing Review SmartForm page in Zipline
- The Status Report [3] form that you complete and then attach to the SmartForm
To request renewal of a Delayed Onset Human Research (DOHR) determination, complete the APPLICATION Determination, Delayed Onset Human Research [6] and follow the instructions on the form.
Submit Continuing Review Report
1. Click Renew or Close in the study workspace
- NOTE: The study must be Approved, Lapsed, or Suspended for this action to be available.
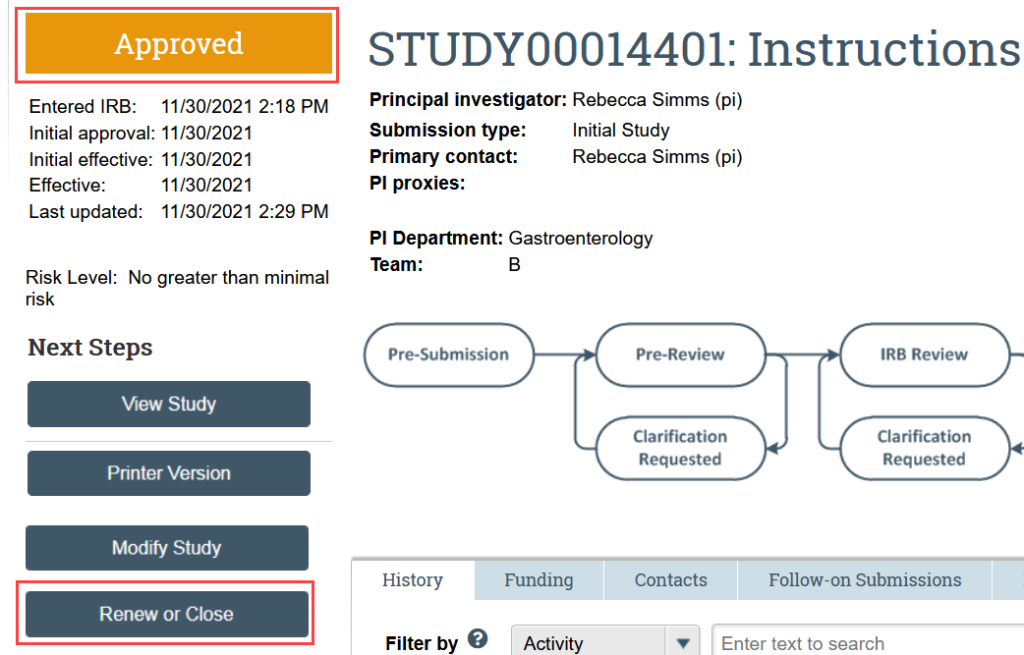
2. Indicate that the purpose of the submission is continuing review and click Continue
3. Complete the Continuing Review/Study Closure SmartForm page
- NOTE: You will be prompted to acknowledge that the study will be closed if the first 4 research milestones are selected.

4. Complete and upload the Status Report form [3]. If your study requires radiation safety approval, you should also attach your radiation safety renewal here.
- Click the link to open the template
- Complete the template and save it to your computer
- Attach the template to the SmartForm, either using drag and drop, or by clicking Add
5. Click Save and Exit to leave the SmartForm and return to the submission workspace
6. Complete the Submit activity (must be completed by the PI or a PI proxy [7])
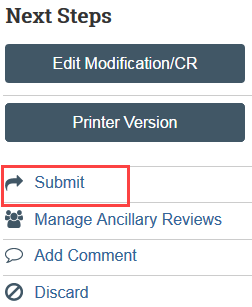
The continuing review transitions to Pre-Review state and is now in HSD’s queue for review.
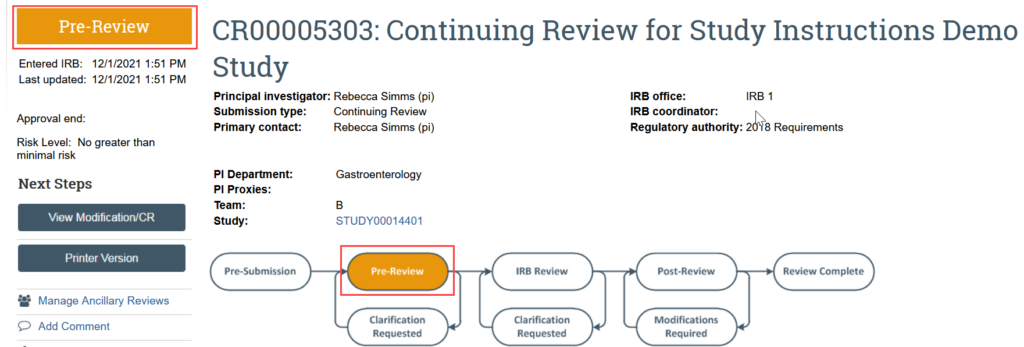
After the IRB has reviewed the application, the PI, any PI proxies, and primary contact will receive a notification that:
- The IRB has approved the renewal or closure; OR
- The IRB requires more information or a change before approving the renewal or closure
See Respond to HSD [8] for more information on submitting responses.
After Continuing Review Approval
After the continuing review is approved, the parent study will be updated. For closure requests, the parent study status is Closed. For renewal requests, the new expiration date is published to the parent study. The PI, PI proxy, and primary contact also receive a notification containing the Continuing Review or Closure approval letter.